Acid Base Balance Part 1 PDF

| Title | Acid Base Balance Part 1 |
|---|---|
| Author | Joshua Rupert |
| Course | Clinical Biochemistry II |
| Institution | University of Ontario Institute of Technology |
| Pages | 3 |
| File Size | 83.3 KB |
| File Type | |
| Total Downloads | 76 |
| Total Views | 177 |
Summary
MLSC-3111, Clinical Biochemistry IIPO 2 and CO 2 Air is comprised of 21% oxygen, 0% carbon dioxide and 78% nitrogen. When we measure pO 2 and pCO 2 in blood it is the only amount of gas in the liquid portion of blood. Partial pressure of gasses is calculated by multiplying 760 mmHg by its percentage...
Description
MLSC-3111, Clinical Biochemistry II PO2 and CO2 -
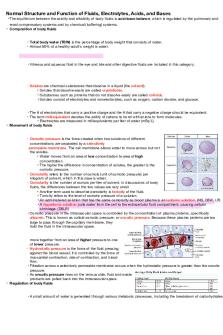
Air is comprised of 21% oxygen, 0.03% carbon dioxide and 78% nitrogen. When we measure pO2 and pCO2 in blood it is the only amount of gas in the liquid portion of blood. Partial pressure of gasses is calculated by multiplying 760 mmHg by its percentage in the atmosphere. Normal cell function requires that ECF pH is maintained between 7.35-7.45. A pH of < 6.8 or > 8.0 is incompatible with life.
Buffer Systems -
Mixtures of weak acids and its salt used to resist changes in pH. Sulfuric acid, phosphoric acid and ketoacids are normally produced by the body (40-80 mmol/L daily). They need to be buffered to avoid pH changes. Bicarbonate Carbonic Acid System, most important and makes up 70% of the body’s buffering capacity. This buffering system is reversible. Hemoglobin, buffers through transporting acidic CO2 to the lungs. Plasma Proteins, minor importance in buffering. The lungs will provide oxygen to RBCs and excrete their CO2. This is the respiratory buffer component. The kidneys remove H+ from the blood via urine and regulates bicarbonate generation and excretion. This is the metabolic buffer component.
Respiratory Control -
-
In the tissues, CO2 is diffused into the RBCs which contain carbonic anhydrase. This results in the CO2 combining with water to make H+ and HCO3- in the RBC. The H+ is buffered with hemoglobin to form reduced hemoglobin. HCO3- diffuses out of the RBCs into the plasma in exchange for Cl- via the chloride shift. Once in the lungs, the reduced hemoglobin absorbs oxygen to release the H+. The lungs will finally take up the H+ from the previously reduced hemoglobin, combining it with HCO3- to create CO2 to be excreted. The oxygenated hemoglobin in the RBCs then leaves the lungs to diffuse their oxygen to the tissues.
Metabolic Control -
When ECF pH is too low, the kidneys will reabsorb more HCO3- and actively excrete hydrogen ions in exchange for Na+. When ECF pH is too high, the kidneys will excrete more HCO3- to reduce hydrogen ion excretion and lower the pH.
MLSC-3111, Clinical Biochemistry II Normal pH -
Normal pH is 7.4 with a reference value of 7.35-7.45. Carbonic acid is not measured and is instead calculated by H2CO3 = 0.03 x pCO2 The equation for pH is pH = pKa + log[([HCO3-]/0.003)/pCO2] Normal pH requires a 20:1 ratio of base to acid (Bicarbonate : pCO2)
Acid-Base Balance Disorders -
-
-
-
-
Acidemia, decreased blood pH. Alkalemia, increased blood pH. We cannot measure the pH of cells, only the blood. Respiratory Acidosis, acidosis caused by impaired breathing resulting in an excess of H+. CO2 cannot be blown off through the lungs, increasing the pCO2 and leaving the bicarbonate levels unchanged. Results in hypercapnia Caused by: o Decreased Respiration Rate, also known as hypoventilation. Occurs in traumatic lung injury, depression of the brains respiratory centre, infections, or drugs. o Decreased Gas Exchange, due to lung disease like pneumonia or lung obstruction. Lack of ventilation results in an increase in pCO2 accumulating in the blood. The increased pCO2 drives the buffer equation to the right, resulting in increased H+ and a decreased pH. Respiratory Alkalosis, a decrease in the H+ due to excessive gas exchange. Decreases the pCO2 while bicarbonate levels remain unchanged. Caused by: o Respiratory Rate Stimulation, also known as hyperventilation. Usually acute and less common than respiratory acidosis and results in hypocapnia. Increased ventilation causes a loss of pCO2 in the blood. Causes the buffer equation to shift to the left, resulting in a loss of H+ and an increased pH. Metabolic Acidosis, an accumulation of H+ resulting in a decreased HCO3concentration. Caused by: o Increased Production of Acid, ketoacidosis, lactic acidosis, toxins. Has a low anion gap. o Decreased Acid Excretion, kidney failure or renal tubular acidosis. (retention of H+ with loss of HCO3-). Has a high anion gap. o Excessive Loss of Bicarbonate, diarrhea (loss of HCO3- through the GI tract) or vomiting of bile (rich in bicarbonate). Has a normal anion gap (compensated with Cl- reabsorption). Metabolic Alkalosis, a decrease in H+ resulting in an increased HCO3- concentration. Caused by: o Loss of H+, severe vomiting (loss of HCl) or loss of kidney function from diuretics. o Ingestion/Infusion of Bicarbonate, overenthusiastic self-administration of antacids especially if taken with milk. Coupled with renal dysfunction.
MLSC-3111, Clinical Biochemistry II
-
-
o Severe Hypokalemia, low potassium will cause the RBC potassium to leave the cell and go into the plasma in exchange for hydrogen ions moving in. Results in low H+ and an increased pH. A loss of H+ results in the equation to move towards the right to compensate. Results in carbonic acid being produced and dissociating into H+ and HCO3-. The H+ stays normal because a lot was lost, but the HCO3- will be in excess. The excess HCO3- will increase the blood pH. pCO2 reflects the respiratory component and HCO3- reflects the metabolic component. pH changes based on pCO2 and HCO3-, but not pO2.
Compensation -
If the disorder is respiratory, the compensation is done through the kidneys through a gain or loss of bicarbonate. If the disorder is metabolic, the compensation is done through the lungs through hyper/hypoventilation. Fully Compensated, pH is returned to the normal range but the original disturbance is still apparent. The compensation will never go above 7.40 in acidosis and will never go below 7.40.
Compensation Mechanisms -
-
-
Respiratory Acidosis, kidneys will increase sodium-hydrogen exchange and bicarbonate retention. Increased bicarbonate will increase pH back to normal. Respiratory Alkalosis, the kidneys will excrete bicarbonate and the loss of bicarbonate will decrease the pH back to normal. Metabolic Acidosis, low pH triggers hyperventilation to decrease the amount of pCO2 in the blood. Loss of pCO2 increases the pH back to normal. Metabolic Alkalosis, high pH triggers hypoventilation to increase the amount of pCO2 in the blood. Gain of pCO2 decreases the pH back to normal. Compensation only occurs when both HCO3- and pCO2 are abnormal. If the HCO3- changes in the same direction as the pH, it is primary compensated metabolic. If the HCO3- changes in a different direction than the pH, it is primary compensated respiratory. Respiratory compensation takes hours to compensate, while metabolic compensation takes days to compensate. In DKA, patients experience significant lipolysis and subsequent ketoacidosis. The increase in ketoacids lowers the AG and requires compensation to increase the pH by excreting CO2. Since this is a metabolic acidosis, the lungs will compensate by hyperventilation which is seen clinically as Kussmaul’s respirations. Kussmaul’s respiration in DKA is symptomatic evidence of respiratory compensation in primary metabolic acidosis in DKA....
Similar Free PDFs

Acid Base Balance Part 1
- 3 Pages

Acid Base Balance Part 2
- 5 Pages

Wk 1 - Acid-Base Balance
- 5 Pages

Acid-Base Balance Summary
- 5 Pages

Acid Base balance, Outline
- 5 Pages

Acid-Base Balance - notes
- 4 Pages

3125 Week 2 Acid-Base Balance
- 10 Pages
Popular Institutions
- Tinajero National High School - Annex
- Politeknik Caltex Riau
- Yokohama City University
- SGT University
- University of Al-Qadisiyah
- Divine Word College of Vigan
- Techniek College Rotterdam
- Universidade de Santiago
- Universiti Teknologi MARA Cawangan Johor Kampus Pasir Gudang
- Poltekkes Kemenkes Yogyakarta
- Baguio City National High School
- Colegio san marcos
- preparatoria uno
- Centro de Bachillerato Tecnológico Industrial y de Servicios No. 107
- Dalian Maritime University
- Quang Trung Secondary School
- Colegio Tecnológico en Informática
- Corporación Regional de Educación Superior
- Grupo CEDVA
- Dar Al Uloom University
- Centro de Estudios Preuniversitarios de la Universidad Nacional de Ingeniería
- 上智大学
- Aakash International School, Nuna Majara
- San Felipe Neri Catholic School
- Kang Chiao International School - New Taipei City
- Misamis Occidental National High School
- Institución Educativa Escuela Normal Juan Ladrilleros
- Kolehiyo ng Pantukan
- Batanes State College
- Instituto Continental
- Sekolah Menengah Kejuruan Kesehatan Kaltara (Tarakan)
- Colegio de La Inmaculada Concepcion - Cebu