CHEM 105 Problem Set 27 PDF
| Title | CHEM 105 Problem Set 27 |
|---|---|
| Course | General College Chemistry |
| Institution | Brigham Young University |
| Pages | 5 |
| File Size | 160.4 KB |
| File Type | |
| Total Downloads | 2 |
| Total Views | 148 |
Summary
Download CHEM 105 Problem Set 27 PDF
Description
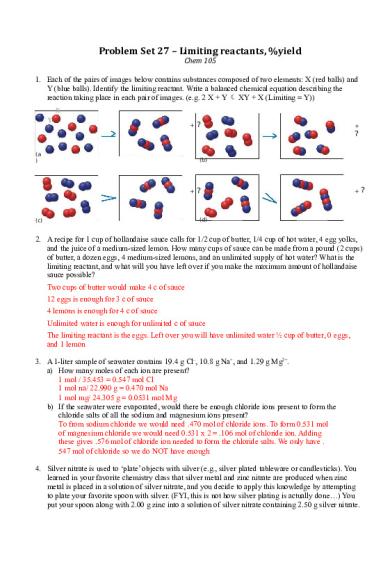
Problem Set 27 – Limiting reactants, %yield Chem 105 1. Each of the pairs of images below contains substances composed of two elements: X (red balls) and Y (blue balls). Identify the limiting reactant. Write a balanced chemical equation describing the reaction taking place in each pair of images. (e.g. 2 X + Y XY + X (Limiting = Y)) X Y
+
?
+
? (a )
(b)
+
?
+?
(d)
(c)
2. A recipe for 1 cup of hollandaise sauce calls for 1/2 cup of butter, 1/4 cup of hot water, 4 egg yolks, and the juice of a medium-sized lemon. How many cups of sauce can be made from a pound (2 cups) of butter, a dozen eggs, 4 medium-sized lemons, and an unlimited supply of hot water? What is the limiting reactant, and what will you have left over if you make the maximum amount of hollandaise sauce possible? Two cups of butter would make 4 c of sauce 12 eggs is enough for 3 c of sauce 4 lemons is enough for 4 c of sauce Unlimited water is enough for unlimited c of sauce The limiting reactant is the eggs. Left over you will have unlimited water ½ cup of butter, 0 eggs, and 1 lemon 3. A 1-liter sample of seawater contains 19.4 g Cl–, 10.8 g Na+, and 1.29 g Mg2+. a) How many moles of each ion are present? 1 mol / 35.453 = 0.547 mol Cl 1 mol na/ 22.990 g = 0.470 mol Na 1 mol mg/ 24.305 g = 0.0531 mol Mg b) If the seawater were evaporated, would there be enough chloride ions present to form the chloride salts of all the sodium and magnesium ions present? To from sodium chloride we would need .470 mol of chloride ions. To form 0.531 mol of magnesium chloride we would need 0.531 x 2 = .106 mol of chloride ion. Adding these gives .576 mol of chloride ion needed to form the chloride salts. We only have . 547 mol of chloride so we do NOT have enough 4. Silver nitrate is used to ‘plate’ objects with silver (e.g., silver plated tableware or candlesticks). You learned in your favorite chemistry class that silver metal and zinc nitrate are produced when zinc metal is placed in a solution of silver nitrate, and you decide to apply this knowledge by attempting to plate your favorite spoon with silver. (FYI, this is not how silver plating is actually done…) You put your spoon along with 2.00 g zinc into a solution of silver nitrate containing 2.50 g silver nitrate.
a) Write a balanced equation for the reaction that (you hope) happens. Zn(s) + 2AGNO3(aq) 2Ag(s) + Zn(NO3) 2(aq) b) Which reactant will run out first? AgNO3 will run out first it is the limiting reactant c) How many grams of silver will be formed, assuming the desired reaction goes to completion? 0.0147 mol AgNO2 x 2 mol / 2 mol AgNo2 x 108 g Ag/ 1 mol Ag = 1.59 g Ag d) Assuming this amount of silver is sufficient to plate the spoon, how many grams of each reagent will it take to replenish your setup and plate another spoon? 0.0147 mol AgNO2 1 mol zn/ 2 mol AgNo2 x 65.4 g / 1 mol = 0.481 Zn used up We don’t need to replace any Zn because we still have 1.519 grams left. We only need 481 grams to plate the spoon. When we put a new spoon in our Zn will still be in excess we will need all of the silver nitrate replaced (2.5 grams) 5. Suppose 75 metric tons of coal that is 3.0% sulfur by mass is burned at a power plant. During combustion the sulfur is converted into SO2. Antipollution scrubbers installed in the smoke stacks of the power plant capture 3.9 metric tons of this SO2. How efficient are the scrubbers in capturing SO2? How many metric tons of SO2 escaped? 2.25 x 10^8 g s x 1 / 32.065 x 1/ 1 mol x 64.064 / 1 x 1 kg/ 1000 g x 1 / 1000 kg = 4.5 met. Ton SO2 Efficiency = 3.9 metric tons captured/ 4.5 metric tons produced x 100 = 87% .60 metric tons of SO2 escaped
6. Baking soda (NaHCO3) is produced on an industrial scale by the Solvay process. A key reaction in the process is NaCl(aq) + NH3(aq) + CO2(aq) + H2O(ℓ) → NaHCO3(s) + NH4Cl(aq) Suppose a reaction vessel initially contains 58.5 kg NaCl, 18.8 kg NH3, and excess CO2 and H2O. If 66 kg NaHCO3 is produced, what is the percent yield? 58500 g NaCl x 1 mol nacl/ 58. 44 g = 1001 mol nacl 1001/1 < 1103/1 nacl limiting 18800 g N H2 x 1 mol/ 17.03 = 1103 mol NH2 58500 g nacl x 1 mol nacl/ 58.44 x 1 mol NaHCO3 / 1 mol NaCl x 84.01 g NAHCO3/ 1 mole x 1 kg/ 1000g = 84.1 kg NaHCO3 l NaCl 66 kg/ 84.1 kg x 100 = 78% 7. An industrial process for producing hydrogen gas is based on the reaction between steam and carbon heated to incandescence, producing a mixture of CO and H2 called synthesis gas, or syngas. a) Write a balanced chemical equation describing the production of syngas. C + H2O CO + H2 b) If a reaction vessel that initially contains 66 kilograms of incandescent carbon and excess steam produces 6.8 kg H2, what is the percent yield? 66 kg c x 1000 g/ 1 kg x 1 mol h2/ 1 mol x 1 kg/ 1000 g = 11 kg 6.8 kg / 11 kg x 100 = 61% yield 8. Commercial manufacturers produce nitric acid (HNO3) by the Ostwald process. The process requires three steps: 4NH3(g) + 5O2(g) → 4NO(g) + 6H2O(g) 2NO(g) + O2(g) → 2NO2(g) 3NO2(g) + H2O (l) → 2HNO3(aq) + NO (g) Begin with 37.0 kg of ammonia (NH3) and an unlimited supply of oxygen and water. a) How many kilograms of oxygen gas will be consumed in the process? 121.7 kg O2 b) How many kilograms of nitric acid will be produced? (i.e., the theoretical yield) 91.3 kg HNO3 c) If the overall process normally has a percent yield of 83.0%, how many kilograms of nitric acid will actually be produced? 75.9 kg HNO3 9. Pound for pound, more sulfuric acid is produced in the world than any other chemical. The formation of H2SO4 is a three-step process: S(s) + O2(g) → SO2(g) 2SO2(g) + O2(g) → 2SO3(g) SO3(g) + H2O(l) → H2SO4(aq) a) If you started with 25.0 kg of sulfur, excess water, and an unlimited supply of oxygen, how many kg of sulfuric acid will be formed if the reaction goes to 100% completion? 76.5 kg H2SO4 b) In real life, the formation of sulfuric acid is an equilibrium process in which the reaction does not go to completion. If you obtain 54.36 kg of sulfuric acid, what is the percent yield? Percent yield = 71.1% 10. Use the following reaction (assume excess carbon monoxide): Fe2O3(s) + 3CO → 2Fe(s) + 3CO2(g) a) If you start with 16g of iron(III) oxide and produce 8.4g of iron metal, what is the % yield? Percent yield = 75% b) If you wanted to produce 100. g of iron metal and you knew the % yield of the reaction was typically 80.%, how much iron(III) oxide would you need to start with? 179 g Fe2O3 11. 185 grams of iron (II) oxide were reacted with 38.0 grams of aluminum as follows: 3 FeO(s) + 2 Al(s) → Al2O3(s) + 3 Fe(g)
a) What was the limiting reactant? Al is the limiting reactant b) How many grams of total product (Al2O3 + Fe) formed if the reaction goes to completion? 190 grams c) The total mass of the reactants is 223 grams. However, this is not the total mass of the products. Why not? Because iron 11 oxide was excess while al was the limiting reactant. The amount of product depends on the limiting reactant. Once the limiting reactant is used up the reaction stops d) If the actual yield of the iron is 105.0 grams, what is the percent yield of iron? Percent yield = 89%...
Similar Free PDFs

CHEM 105 Problem Set 27
- 5 Pages

CHEM 105 Problem Set 5
- 6 Pages

CHEM 105 Problem Set 12
- 2 Pages

CHEM 105 Problem Set 31
- 2 Pages

CHEM 105 Problem Set 21
- 2 Pages

CHEM 105 Problem Set 20
- 4 Pages

CHEM 105 Problem Set 10
- 4 Pages

CHEM 105 Problem Set 35
- 4 Pages

CHEM 105 Problem Set 23
- 5 Pages

CHEM 105 Problem Set 25
- 4 Pages

CHEM 105 Problem Set 28
- 5 Pages

Problem Set 2 - Chem 105
- 2 Pages

Problem+set+4 KEY CHEM+4320
- 6 Pages
Popular Institutions
- Tinajero National High School - Annex
- Politeknik Caltex Riau
- Yokohama City University
- SGT University
- University of Al-Qadisiyah
- Divine Word College of Vigan
- Techniek College Rotterdam
- Universidade de Santiago
- Universiti Teknologi MARA Cawangan Johor Kampus Pasir Gudang
- Poltekkes Kemenkes Yogyakarta
- Baguio City National High School
- Colegio san marcos
- preparatoria uno
- Centro de Bachillerato Tecnológico Industrial y de Servicios No. 107
- Dalian Maritime University
- Quang Trung Secondary School
- Colegio Tecnológico en Informática
- Corporación Regional de Educación Superior
- Grupo CEDVA
- Dar Al Uloom University
- Centro de Estudios Preuniversitarios de la Universidad Nacional de Ingeniería
- 上智大学
- Aakash International School, Nuna Majara
- San Felipe Neri Catholic School
- Kang Chiao International School - New Taipei City
- Misamis Occidental National High School
- Institución Educativa Escuela Normal Juan Ladrilleros
- Kolehiyo ng Pantukan
- Batanes State College
- Instituto Continental
- Sekolah Menengah Kejuruan Kesehatan Kaltara (Tarakan)
- Colegio de La Inmaculada Concepcion - Cebu


