Chemistry Nucleophilic Aromatic Substitution Experiment PDF
| Title | Chemistry Nucleophilic Aromatic Substitution Experiment |
|---|---|
| Author | Martha Perez |
| Course | Chemistry Internship |
| Institution | The University of Texas Rio Grande Valley |
| Pages | 4 |
| File Size | 154.5 KB |
| File Type | |
| Total Downloads | 95 |
| Total Views | 150 |
Summary
one...
Description
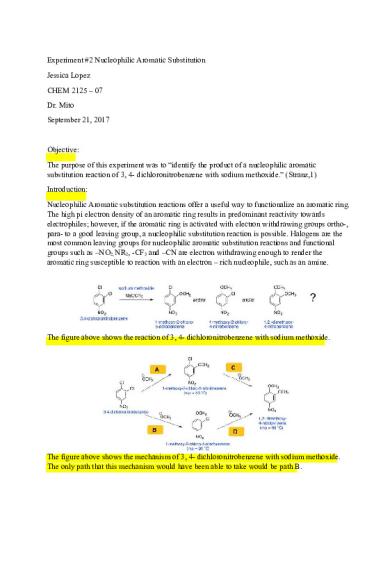
Experiment #2 Nucleophilic Aromatic Substitution Jessica Lopez CHEM 2125 – 07 Dr. Mito September 21, 2017
Objective: The purpose of this experiment was to “identify the product of a nucleophilic aromatic substitution reaction of 3, 4- dichloronitrobenzene with sodium methoxide.” (Stranz,1) Introduction: Nucleophilic Aromatic substitution reactions offer a useful way to functionalize an aromatic ring. The high pi electron density of an aromatic ring results in predominant reactivity towards electrophiles; however, if the aromatic ring is activated with electron withdrawing groups ortho-, para- to a good leaving group, a nucleophilic substitution reaction is possible. Halogens are the most common leaving groups for nucleophilic aromatic substitution reactions and functional groups such as –NO2, NR2, -CF3 and –CN are electron withdrawing enough to render the aromatic ring susceptible to reaction with an electron – rich nucleophile, such as an amine.
The figure above shows the reaction of 3, 4- dichloronitrobenzene with sodium methoxide.
The figure above shows the mechanism of 3, 4- dichloronitrobenzene with sodium methoxide. The only path that this mechanism would have been able to take would be path B.
Materials: The equipment used in this experiment: -
50 mL round bottom flask 150 mL beaker 400 mL beaker Graduated cylinder Thermometer Condenser Rubber tubing Hot plate Separatory funnel Ice bath Buchner funnel Flask Filter paper Spatula DigiMelt Capillaries Water/methanol solution 0.166 3, 4-dichloronitrobenzene ( C6 H 3 Cl2 NO2 ) 15 mL methanol ( CH 4 / MeOH ¿ 10 mL 25%sodium methoxide in metanol (NaOMe)
Procedure: 1. We gathered our materials to set up the reflux apparatus including the stand, a rubber clamp, and 50 mL round bottom flask, condenser, plastic clamp, and a hot plate. We then gathered our chemicals 15 mL of methanol, 10 mL of 25% NaOMe, added to that two boiling chips and the flask was then placed onto the hot plate. We allowed our solution to begin to boil then started the timer for one hour. 2. We prepared an ice bath in a 400 mL beaker around the 45 minute mark to prepare for the next part of the experiment. 3. We waited for the hour to be up and after that we set the round bottom flask aside allowed it to cool for about 10 minutes then adding it to an empty 150 mL beaker. We then added 25 mL of water to our product and allowed it to precipitate. 4. When the water was added it was for the most part fully precipitated but it needed a little more so we proceeded to add it to the ice bath for around 5 minutes to fully precipitate the liquid. After it was fully precipitated we assembled our vacuum to filter the precipitate out. We put the filter paper onto the funnel, adding a bit of water so that way it none of our product would go into the flask.
5. Next the 60:40 water/methanol solution was prepared with 6 mL‘s of water and 4 mL’s of methanol into a 10 mL graduated cylinder. We then poured our product into the vacuum and washed it out with the water/methanol solution. 6. Allowing our product to dry on the filter paper, we then weighed the mass of our product on a watch glass. We set up the digimelt was plugged in and we put our product into two capillaries and the meting point was recorded. Results: Moles of 3, 4- Dichloronitrobenzene: 0.166g 3, 4- dichloronitrobenzene
1 mol 192 g/mol
= 0.00086 mol of 3, 4- dichloronitrobenzene
3, 4-dichloronitrobenzene
Theoretical yield: (mass of the 3,4-DCNB/molar mass of 3,4- DCNB) * (1 mol product/1mol of 3, 4DCNB*Molar mass of sodium methoxide) (0.166g 3,4-DCNB/192 g 3, 4- DCNB)*( 1 mol of product/1 mol of 3,4- DCNB*54.02 g/mol NaOMe) = 0.047 Percent yield: (Mass of recovered product/ Theoretical yield) * 100% (0.173g /0.047)*100% = 368 % Discussion: Our experiment was successful due to the fact that we obtained a great amount of product and I believe that the modification to the experiment made it run a lot smoother than if we had to use a sand bath, and also because at times the lab manual is unclear. Our only downfall was not knowing the correct value for the temperature in the beginning of the experiment because in the lab manual there were two temperatures that were stated. Conclusion: The objective of this experiment was to “identify the product of a nucleophilic aromatic substitution reaction of 3, 4-dichloronitrobenzene with sodium methoxide.” (Stranz, 1). Our experiment was considered successful, because we were able to obtain a great amount of product and as well find the melting point of our product using the DigiMelt which was between the temperatures of 58 - 98 degrees celcius. References:
Stranz, Michael. Signature Lab Series. Cengage Learning 2008 Mito, Shizue. Pre Lab Notes...
Similar Free PDFs
Popular Institutions
- Tinajero National High School - Annex
- Politeknik Caltex Riau
- Yokohama City University
- SGT University
- University of Al-Qadisiyah
- Divine Word College of Vigan
- Techniek College Rotterdam
- Universidade de Santiago
- Universiti Teknologi MARA Cawangan Johor Kampus Pasir Gudang
- Poltekkes Kemenkes Yogyakarta
- Baguio City National High School
- Colegio san marcos
- preparatoria uno
- Centro de Bachillerato Tecnológico Industrial y de Servicios No. 107
- Dalian Maritime University
- Quang Trung Secondary School
- Colegio Tecnológico en Informática
- Corporación Regional de Educación Superior
- Grupo CEDVA
- Dar Al Uloom University
- Centro de Estudios Preuniversitarios de la Universidad Nacional de Ingeniería
- 上智大学
- Aakash International School, Nuna Majara
- San Felipe Neri Catholic School
- Kang Chiao International School - New Taipei City
- Misamis Occidental National High School
- Institución Educativa Escuela Normal Juan Ladrilleros
- Kolehiyo ng Pantukan
- Batanes State College
- Instituto Continental
- Sekolah Menengah Kejuruan Kesehatan Kaltara (Tarakan)
- Colegio de La Inmaculada Concepcion - Cebu















