Covalent Bonds Gizmo 5 mark test answers PDF
| Title | Covalent Bonds Gizmo 5 mark test answers |
|---|---|
| Author | Dhruv Vm |
| Course | Organic Chemistry |
| Institution | Indian Institute of Technology Indore |
| Pages | 5 |
| File Size | 281.2 KB |
| File Type | |
| Total Downloads | 21 |
| Total Views | 156 |
Summary
Covalent Bonds Gizmo 5 mark test answers...
Description
9/2/2021
Covalent Bonds Gizmo : ExploreLearning
ASSESSMENT QUESTIONS:
Print Page
DHRUV V M
Q1
Q2
Q3
Q4
Q5
SCORE
Your Results saved
5/5
for class Science
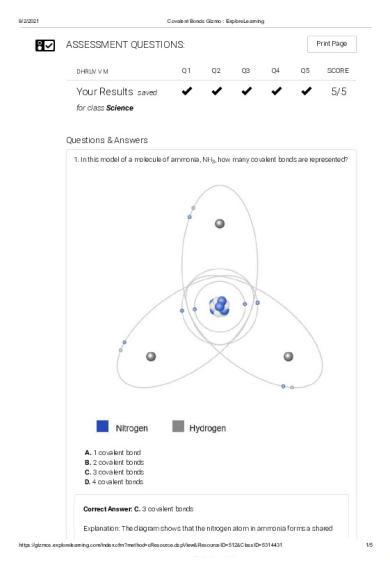
Questions & Answers 1. In this model of a molecule of ammonia, NH3, how many covalent bonds are represented?
A. 1 covalent bond B. 2 covalent bonds C. 3 covalent bonds D. 4 covalent bonds
Correct Answer: C. 3 covalent bonds Explanation: The diagram shows that the nitrogen atom in ammonia forms a shared https://gizmos.explorelearning.com/index.cfm?method=cResource.dspView&ResourceID=512&ClassID=5314431
1/5
9/2/2021
Covalent Bonds Gizmo : ExploreLearning
orbit for two electrons with each hydrogen atom. Each pair of electrons in a shared orbit represents one covalent bond. In this case, there are three pairs of shared electrons, or three covalent bonds.
You answered this question correctly!
2. The image below shows two nitrogen atoms. For these two atoms to form a stable molecule, N2, how many electrons would have to be part of covalent bonds?
A. 0 electrons B. 2 electrons C. 4 electrons D. 6 electrons
Correct Answer: D. 6 electrons Explanation: A single nitrogen atom has ve electrons in its outer energy level to start, and will be most stable with an octet, or 8 electrons in its outer level. When forming a covalent molecule with another nitrogen atom, each atom shares 3 electrons, forming 3 covalent bonds with 2 electrons each. So, there are 6 electrons involved in covalent bonds. This can be veried by looking at the Lewis diagram of a nitrogen molecule shown below. (Each bar represents a covalent bond.)
You answered this question correctly!
3. How many covalent bonds are there in one molecule of carbon dioxide, CO2 ?
https://gizmos.explorelearning.com/index.cfm?method=cResource.dspView&ResourceID=512&ClassID=5314431
2/5
9/2/2021
Covalent Bonds Gizmo : ExploreLearning
A. 0 covalent bonds B. 2 covalent bonds C. 4 covalent bonds D. 6 covalent bonds
Correct Answer: C. 4 covalent bonds Explanation: Carbon dioxide, CO2, is made up of one carbon atom covalently bonded with two oxygen atoms. Carbon starts with 4 valence electrons, needing to share 4 more electrons to form a stable octet. Meanwhile each oxygen atom starts with 6 valence electrons and needs to share 2 additional atoms to be stable. So, the carbon atom forms two bonds with each of the two oxygen atoms, for a total of 4 covalent bonds. Each covalent bond is represented by a dash in the Lewis diagram.
You answered this question correctly!
4. Bromine is a halogen, one of the elements in the same column of the periodic table of elements as uorine. Like uorine, one bromine atom can form a diatomic covalent molecule with another bromine atom. Which of the following images represents a stable molecule of bromine, Br2 ? (Note: Inner electrons have been hidden.)
A. Diagram A B. Diagram B C. Diagram C D. Diagram D
https://gizmos.explorelearning.com/index.cfm?method=cResource.dspView&ResourceID=512&ClassID=5314431
3/5
9/2/2021
Covalent Bonds Gizmo : ExploreLearning
Correct Answer: B. Diagram B Explanation: Each bromine atom starts with 7 electrons in its outer energy level. This means that together, they will form one covalent bond that shares two electrons between them. In the second snapshot, you can see that each atom has 6 electrons orbiting in its own outer energy level and that there is a pair of electrons in the shared orbit. Each atom is orbited by 8 electrons, and the molecule is stable.
You answered this question correctly!
5. Hydrogen cyanide is poisonous liquid that has a faint, almond-like smell. One molecule of hydrogen cyanide (HCN) is made up of one hydrogen atom, one carbon atom, and one nitrogen atom, shown below. Which of the answer choices shows the correct Lewis dot diagram for hydrogen cyanide?
A. Diagram A B. Diagram B C. Diagram C D. Diagram D
Correct Answer: D. Diagram D Explanation: To be stable, a hydrogen atom needs to have 2 electrons in orbit around its nucleus. In this diagram, the hydrogen atom is sharing two electrons in a covalent bond with the carbon atom. This is represented by a line between the H and the C. The carbon atom has four covalent bonds, one with hydrogen and three with nitrogen (the three lines connecting the C to the N). Because each covalent bond has two https://gizmos.explorelearning.com/index.cfm?method=cResource.dspView&ResourceID=512&ClassID=5314431
4/5
9/2/2021
Covalent Bonds Gizmo : ExploreLearning
electrons, the carbon atom has a full, stable complement of 8 electrons in the outer orbit around its nucleus. Nitrogen has two of its own electrons, as represented by the dots to the right of the N, and 6 electrons shared in covalent bonds with the carbon atom. So, nitrogen also effectively has a total of 8 electrons in its outer energy level. This means all the atoms in the diagram are stable.
You answered this question correctly!
https://gizmos.explorelearning.com/index.cfm?method=cResource.dspView&ResourceID=512&ClassID=5314431
5/5...
Similar Free PDFs

Covalent Bonding Gizmo Answers
- 7 Pages

MARK 3000 Chapter 5 Test
- 75 Pages

Air Track gizmo Answers
- 5 Pages

Moles Gizmo Answers
- 8 Pages

Ionic Bonding Gizmo Answers
- 6 Pages

DNA Gizmo - Answers
- 5 Pages

P H Gizmo Answers
- 3 Pages

Moles Gizmo Answers
- 8 Pages

Photosynthesis Lab Gizmo Answers
- 5 Pages

Digestive system gizmo answers
- 9 Pages
Popular Institutions
- Tinajero National High School - Annex
- Politeknik Caltex Riau
- Yokohama City University
- SGT University
- University of Al-Qadisiyah
- Divine Word College of Vigan
- Techniek College Rotterdam
- Universidade de Santiago
- Universiti Teknologi MARA Cawangan Johor Kampus Pasir Gudang
- Poltekkes Kemenkes Yogyakarta
- Baguio City National High School
- Colegio san marcos
- preparatoria uno
- Centro de Bachillerato Tecnológico Industrial y de Servicios No. 107
- Dalian Maritime University
- Quang Trung Secondary School
- Colegio Tecnológico en Informática
- Corporación Regional de Educación Superior
- Grupo CEDVA
- Dar Al Uloom University
- Centro de Estudios Preuniversitarios de la Universidad Nacional de Ingeniería
- 上智大学
- Aakash International School, Nuna Majara
- San Felipe Neri Catholic School
- Kang Chiao International School - New Taipei City
- Misamis Occidental National High School
- Institución Educativa Escuela Normal Juan Ladrilleros
- Kolehiyo ng Pantukan
- Batanes State College
- Instituto Continental
- Sekolah Menengah Kejuruan Kesehatan Kaltara (Tarakan)
- Colegio de La Inmaculada Concepcion - Cebu





