Lastprelabyass - Lab for organic Chemistry ii PDF
| Title | Lastprelabyass - Lab for organic Chemistry ii |
|---|---|
| Author | Sarah DeRosa |
| Course | Organic Chemistry Ii |
| Institution | Cleveland State University |
| Pages | 3 |
| File Size | 171.5 KB |
| File Type | |
| Total Downloads | 75 |
| Total Views | 135 |
Summary
Lab for organic Chemistry ii ...
Description
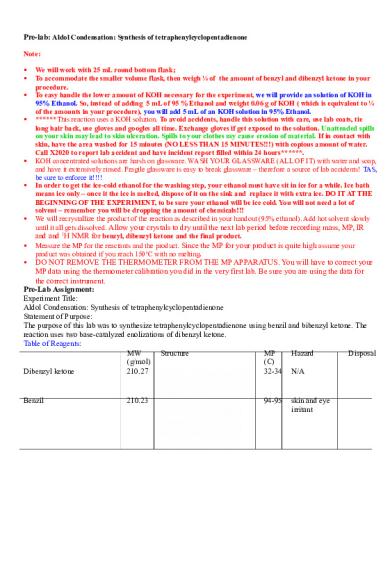
Pre-lab: Aldol Condensation: Synthesis of tetraphenylcyclopentadienone Note:
We will work with 25 mL round bottom flask; To accommodate the smaller volume flask, then weigh ¼ of the amount of benzyl and dibenzyl ketone in your procedure. To easy handle the lower amount of KOH necessary for the experiment, we will provide an solution of KOH in 95% Ethanol. So, instead of adding 5 mL of 95 % Ethanol and weight 0.06 g of KOH ( which is equivalent to ¼ of the amounts in your procedure), you will add 5 mL of an KOH solution in 95% Ethanol. ******This reaction uses a KOH solution. To avoid accidents, handle this solution with care, use lab coats, tie long hair back, use gloves and googles all time. Exchange gloves if get exposed to the solution. Unattended spills on your skin may lead to skin ulceration. Spills to your clothes my cause erosion of material. If in contact with skin, have the area washed for 15 minutes (NO LESS THAN 15 MINUTES!!!) with copious amount of water. Call X2020 to report lab accident and have incident report filled within 24 hours******. KOH concentrated solutions are harsh on glassware. WASH YOUR GLASSWARE (ALL OF IT) with water and soap, and have it extensively rinsed. Fragile glassware is easy to break glassware – therefore a source of lab accidents! TAS, be sure to enforce it!!!! In order to get the ice-cold ethanol for the washing step, your ethanol must have sit in ice for a while. Ice bath means ice only – once it the ice is melted, dispose of it on the sink and replace it with extra ice. DO IT AT THE BEGINNING OF THE EXPERIMENT, to be sure your ethanol will be ice cold. You will not need a lot of solvent – remember you will be dropping the amount of chemicals!!! We will recrystallize the product of the reaction as described in your handout (95% ethanol). Add hot solvent slowly until it all gets dissolved. Allow your crystals to dry until the next lab period before recording mass, MP, IR and and 1H NMR for benzyl, dibenzyl ketone and the final product. Measure the MP for the reactants and the product. Since the MP for your product is quite high assume your product was obtained if you reach 150oC with no melting.
DO NOT REMOVE THE THERMOMETER FROM THE MP APPARATUS. You will have to correct your MP data using the thermometer calibration you did in the very first lab. Be sure you are using the data for the correct instrument. Pre-Lab Assignment: Experiment Title: Aldol Condensation: Synthesis of tetraphenylcyclopentadienone Statement of Purpose: The purpose of this lab was to synthesize tetraphenylcyclopentadienone using benzil and bibenzyl ketone. The reaction uses two base-catalyzed enolizations of dibenzyl ketone. Table of Reagents: MW Structure MP Hazard Disposal (g/mol) (C) Dibenzyl ketone 210.27 32-34 N/A
Benzil
210.23
94-95
skin and eye irritant
Tetraphenylcyclopentadienone 384.47
Potassium Hydroxide
56.11
95% ethanol
46.07
217220
BP— 78.3
N/A
skin irritant, serious eye damage, corrosive skin irritant, eye irritant
Procedure Outline: Add .2625g of benzil and .2625g of dibenzyl ketone with 5mL of 95% ethanol in a 100mL round-bottom flask Add .23g of KOH and a stir bar Boil and reflux for 15 mins Mixture should turn purply Cool the mixture to room temperature then ice bath Collect the purply crystals via vacuum filtration Wash crystals with 10mL of cold 95% ethanol Allow product to air dry Recrystallization: Place solid in a 10-25ml Erlenmeyer flask Use a Pasteur pipette to add only enough solid to cover the crystals Heat the contents to boiling The add additional solid drop by drop Continue until fully dissolved Slowly cool the solution to produce purer crystals Allow solution to cool to room temperature Assemble filtration apparatus and collect crystals Recrystallization works to remove impurities in a compound Pre-lab questions: 1. (1.5 points) What is the difference between Aldol Addition and Aldol condensation reaction? An aldol condensation reaction combines two molecules by forming a new C—C bond while removing a small molecule, usually water. In an aldol addition reaction, one molecule of a carbonyl compound reacts as a nucleophile after a proton is removed from the alpha carbon and adds to the eletrophillic carbonyl carbon of a second molecule. 2. (0.5 point) What is the function of KOH in this reaction? KOH acts to supply the reaction with the hydroxide ion that functions to remove a proton from the alpha carbon. 3. (1 point) What is the Molarity of KOH solution used in this experiment? Show NEAT work. KOH molarity .06g(1mol/56.09g)=.0011mol/005L=.214M 4. (2 points) What is the limiting reagent for this reaction? How much you are expected to obtain of tetraphenylcyclopentanone?
Dibenzyl ketone Limiting .2625g(1mol/210.27)(1mol/1mol)=.0012483 mol Benzil .2625g(1mol/210.23)(1mol/1mol)= .0012486 Tetraphenylcyclopentanone .0012486mol(384.27g/mol)=.48g 5. (2.0 points) Let’s consider possible side products for this reaction. Is it possible for Benzil to form an enol? Can the enolized dibenzyl ketone attacks another dibenzyl ketone for a side product? It is not possible for a benzil to form an enol since there are no hydrogens available for OH to attack and take so no C=C bond can form. It is possible for an enolized dibenzyl ketone for a side product. Citation: Analytical, Biology, Chemistry & Materials Science products and services. http://www.sigmaaldrich.com/ (accessed Nov 13 2017). Mohrig, Jerry R., et al. Laboratory Techniques in Organic Chemistry: Supporting Inquiry-Driven Experiments. 4th ed., W.H.Freeman, 2014. Pedersen, S.; Myers, A.; Gilbert, J.C.; Martin, S.F.; Williamson, K.L.; Masters, K.M.; Hill, N.; Esselman, B.; Organic Chemistry lab I/ II; (Chem lab 336/337); Custom Edition For Cleveland State University; Cengage Learning; 2015; ISBN:1-305-76438-2...
Similar Free PDFs

Lab report for organic chemistry
- 4 Pages

Organic II Chemistry 332
- 9 Pages

Organic Chemistry Lab 6
- 15 Pages

Organic chemistry lab 7
- 9 Pages

LAB Report Organic Chemistry
- 10 Pages

organic chemistry lab 6
- 7 Pages

Organic Chemistry Lab 1
- 14 Pages

Organic Chemistry Lab 1
- 5 Pages

Organic Chemistry II - QUIZ 1
- 2 Pages

Organic Chemistry Lab Report #7
- 6 Pages

Organic Chemistry Lab Report #6
- 6 Pages

Organic Chemistry Lab Report #2
- 9 Pages

Organic Chemistry Lab Report #6
- 6 Pages
Popular Institutions
- Tinajero National High School - Annex
- Politeknik Caltex Riau
- Yokohama City University
- SGT University
- University of Al-Qadisiyah
- Divine Word College of Vigan
- Techniek College Rotterdam
- Universidade de Santiago
- Universiti Teknologi MARA Cawangan Johor Kampus Pasir Gudang
- Poltekkes Kemenkes Yogyakarta
- Baguio City National High School
- Colegio san marcos
- preparatoria uno
- Centro de Bachillerato Tecnológico Industrial y de Servicios No. 107
- Dalian Maritime University
- Quang Trung Secondary School
- Colegio Tecnológico en Informática
- Corporación Regional de Educación Superior
- Grupo CEDVA
- Dar Al Uloom University
- Centro de Estudios Preuniversitarios de la Universidad Nacional de Ingeniería
- 上智大学
- Aakash International School, Nuna Majara
- San Felipe Neri Catholic School
- Kang Chiao International School - New Taipei City
- Misamis Occidental National High School
- Institución Educativa Escuela Normal Juan Ladrilleros
- Kolehiyo ng Pantukan
- Batanes State College
- Instituto Continental
- Sekolah Menengah Kejuruan Kesehatan Kaltara (Tarakan)
- Colegio de La Inmaculada Concepcion - Cebu


