Chemical Biology 2L03 Lab #2B SDS-PAGE PDF

| Title | Chemical Biology 2L03 Lab #2B SDS-PAGE |
|---|---|
| Course | Chemical Biology Laboratory I |
| Institution | McMaster University |
| Pages | 9 |
| File Size | 382.2 KB |
| File Type | |
| Total Downloads | 100 |
| Total Views | 132 |
Summary
Lab details for the second lab (part B) in ChemBio 2L03. This lab uses SDS-PAGE to examine the purity of the crude albumin sample acquired in Lab 1....
Description
CHEMBIO2L03 LAB #2B
SDS-PAGE
LAB #2B ANALYSIS OF PROTEINS BY SDS-PAGE WITH RESPECT TO SUBUNIT COMPOSITION AND MOLECULAR WEIGHT OVERVIEW This experiment is comprised of two parts, which will be performed in tandem. In one part, you will purify the crude extract by size exclusion chromatography (SEC). This process will determine the molecular weight of ovalbumin, and purify the majority of the protein sample for future use. In the other half of this experiment you will have the opportunity to perform sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE), a common procedure for analyzing proteins with respect to purity, molecular weight, and subunit composition on a analytical scale, using minute amounts of sample. Marker Protein, Unknown Sample, Standard Proteins SDS-PAGE
Coomassie Staining
Destaining Gel Documentation
Rf Determination
Calibration Plot
MW DETERMINATION & UNKNOWN IDENTIFICATION
INTRODUCTION In 1948 the Nobel Prize in Chemistry was awarded to Arne Tiselius for his work on the development of electrophoresis. Electrophoresis is a form of chromatography in which charged particles dispersed in liquid migrate under the influence of an electric field. Separation varies with: • • • • • •
number of charges per particle distance between electrodes size and shape of particles viscosity of electrolyte solubility of solute time allowed for separation
• • • • •
sign of charge voltage across apparatus temperature concentration of electrolyte adsorption of solute into the gel
Various stationary phases suitable for electrophoresis are: paper, cellulose-acetate, agar gel, starch gel, starch grains, and polyacrylamide gel. Polyacrylamide gel electrophoresis (PAGE) is the latest to be developed and is among the most useful and most versatile because it readily resolves a wide array of proteins and nucleic acids. Page 1
CHEMBIO2L03 LAB #2B
SDS-PAGE
Polyacrylamide gels are formed by polymerization of acrylamide (monomer) and N,N’-methylene-bisacrylamide (cross-linker). The acrylamide monomer and cross-linker are stable by themselves or when mixed together in solution, but polymerize readily in the presence of a radical generating system. In the most commonly used method, the radical initiator, ammonium persulfate (APS), is added along with a N,N,N’,N’-tetramethylethylenediamine (TEMED) catalyst. These two components, in the presence of the monomer, cross-linker, and appropriate buffer, generate the radicals needed to induce polymerization. Most of the gel consists of a linear polymer of the acrylamide. The small (2-5%) amount of bis-acrylamide acts as a cross-linker between the linear chains, creating a three dimensional network of polymer strands, with pores or channels of fairly uniform size. The porosity of a gel acts as a molecular sieve in which macromolecules of various sizes can be separated. The size of the pores is dependent on the amount of bis-acrylamide and is referred to as the concentration of cross-linker, %C (Equation 1). %C =
[mass bis-acrylamide/(mass acrylamide + mass bis-acrylamide)] X 100%
(Equation 1)
The higher the concentration of cross-linker, the slower the separation due to decreased pore size. The degree of separation also depends on the total concentration of the monomer and cross-linker. This is referred to as the total concentration, %T (Equation 2), and usually has a range of 7 to 20%. The higher the %T, the slower the separation due to increased gel density. %T =
[(mass acrylamide + mass bis-acrylamide)/total volume] X 100% (Equation 2)
In a fast gel (low %T), molecules must travel across a greater distance to achieve separation since all of the species will tend to be near the front and the distance between the bands will be minimal. In contrast, a slower gel (high %T) will result in greater resolution, but the clarity of the bands will be reduced due to band broadening. The use of a discontinuous system incorporates both a fast (stacking gel) and a slow (resolving gel) gel, which allows for greater separation. The stacking gel (low %T) allows molecules of similar size to “stack” into bands of similar size, which are then resolved on the separating gel (high %T). SDS-PAGE is a modification of PAGE that is routinely used to determine the molecular weight of a protein as well as its subunit composition (i.e., number and size of subunits). The protein preparation is treated with an excess of soluble thiol (usually 2-mercaptoethanol or DTT) and SDS. Under these conditions the thiol reduces all disulfide bonds (–S–S–) present within and/or between peptide units, while the SDS (an anionic, denaturing detergent) binds to all regions of the protein and disrupts noncovalent inter- and intramolecular interactions. These two components result in total denaturation of the proteins in the sample, yielding unfolded, highly anionic (negatively charged) polypeptide chains. The charge-to-mass ratio of all of the proteins in the sample is approximately the same, which allows separation of proteins based on size. Every polyacrylamide gel will be slightly different in terms of porosity and rigidity, so there is no direct method for correlating the distance travelled through the gel to the molecular weight of an unknown protein. For this reason, it is necessary to resolve standard proteins of known molecular weight on the gel in order to “calibrate” the molecular weight scale. An example of such an approach can be found in reference #3.
Page 2
CHEMBIO2L03 LAB #2B
SDS-PAGE
SUPPLIES/REAGENTS Running Buffer • 1X Tris-Glycine SDS Running buffer [25 mM Tris-HCl pH 8.3, 192 mM glycine, 0.1% SDS] Laemmli Sample Buffer (LSB) • 62.5 mM Tris-HCl pH 6.8, 25% glycerol, 2% SDS, 0.01% bromophenol blue, 350 mM DTT FroggaBio BLUeye Prestained Protein Ladder (10-245 kDa) • Catalogue #PM007-0500 Bio-Rad® 4-20% Mini-PROTEAN® TGXTM Precast Gel • Catalogue #456-1093 Coomassie Blue Staining Solution • 40% methanol, 10% glacial acetic acid, 0.25% (w/v) Coomassie Brilliant Blue R-250 in water Destaining solution • 40% methanol, 10% glacial acetic acid in water Protein Standard Solutions • The following are commercially bought proteins that have been individually prepared in 1X PBS (phosphate buffered saline) buffer. Underlining = label that will be on the tube you are given. • β-galactosidase (from E. coli; β-gal), Bovine Serum Albumin (BSA), Lactate Dehydrogenase (from porcine heart, LDH), Ovalbumin (from chicken egg white, oval), β-casein (from bovine milk, βcas), and Cytochrome C (from equine heart, cytoC) Crude Ovalbumin Protein Sample • 10 mg/mL ovalbumin from Lab #1 (dissolve in supplied 1X PBS buffer) Unknown Protein Sample (~1 mg/mL concentration) • Tube will be labelled with an identifier (e.g., SDS 1021) Bio-Rad Mini-PROTEAN® Tetra Cell Electrophoresis Module Assembly Power Supply
In the “Other” section of your lab notebook: ➢ Construct a table listing the molecular weights of all of the proteins used in this lab and list the order in which you expect them to appear on the gel (top of the gel → bottom of gel). Make sure to properly reference the molecular weight values. ➢ Look up the information on the protein marker; print a copy of the marker and paste it into your lab notebook. Make sure that each protein band is clearly labelled and the molecular weight is indicated.
Page 3
CHEMBIO2L03 LAB #2B
SDS-PAGE
➢ Prior to the lab, watch the following online video tutorial that illustrates how to set up and run a Mini-PROTEAN ® TGX™ Precast Gel in a Mini-PROTEAN Tetra Cell. In point form, summarize five key points from the video. https://www.bio-rad.com/en-ca/life-science-research/support/tutorials
EXPERIMENTAL Safety Note: Wear gloves and a lab coat. Although not harmful, Coomassie Blue dye will stain skin and clothing. Caution: the power supply is high voltage. Note: i) Dispose your pipette tips first into a beaker and then empty them into the garbage. ii) Keep all protein samples on ice. PART 1: Bio-Rad® Ready Gel Precast Gel Preparation 1. Remove the Ready Gel from the storage pouch. 2. Gently remove the comb and rinse the wells thoroughly with a small amount of running buffer. • Position both thumbs on the ridges of the comb. Remove the comb by pushing upward in one smooth continuous motion. 3. Pull the green tape gently to remove it from the bottom of the cassette. 4. NOTE: If only one gel is to be run, use the mini cell buffer dam in place of a gel cassette. PART 2: Bio-Rad Mini-PROTEAN® Tetra Cell Electrophoresis Module Assembly
Figure 1. Overview of the Bio-Rad electrophoresis module. There are three main parts to this system:
Page 4
CHEMBIO2L03 LAB #2B
SDS-PAGE
i) a lid that connects to a power supply, ii) an electrode assembly that holds the gels in place, and iii) a mini tank to which the electrode assembly is placed. The mini tank and lid combine to fully enclose the inner chamber during electrophoresis. As a built in safety feature, the lid cannot be removed without disrupting the electrical circuit. Refer to reference #2 for more information.
NOTE: Two students will share one electrophoresis module. 1. Place a gel cassette sandwich into the slots at the bottom of each side of the electrode assembly. Be sure the short plate of the gel cassette sandwich faces inward. Secure the gels in place by closing the two green side clamps. Refer to Figure 2. 2. Lower the inner chamber assembly into the mini tank. Make sure the electrodes are properly oriented to connect when the lid is secured. Fill the inner chamber first with running buffer until the level reaches just under the top of the taller plate. CHECK FOR LEAKAGE NOW! There should be absolutely no leakage of buffer around the gasket. If there is leakage, disassemble the cassette and start the assembly again. 3. Add running buffer to the outer chamber until the level reaches the appropriate line marked on the tank.
Figure 2. Assembling the Mini-PROTEAN Tetra Cell Electrophoresis Module. 1. Set the clamping frame to the open position on a flat surface. 2. Place the gel sandwiches (short plate facing inward) onto the gel supports. 3. Using one hand, gently pull both gels towards each other, making sure they rest firmly and squarely against the green gaskets that are built into the clamping frame. Make sure that the short plates sit just below the top of the green gasket. 4. While gently squeezing the gel sandwiches against the green gaskets with one hand (keeping constant pressure so the gels are held in place, slide the green arms of the clamping frame over the gels, locking them in place. NOTE: do not attempt to lock the green arms of the clamping frame without first ensuring that the gel sandwiches are perfectly aligned and stabilized. Refer to reference 2 for more information.
Page 5
CHEMBIO2L03 LAB #2B
SDS-PAGE
PART 3: Sample Preparation ➢ Before you begin preparing your samples and standards, set up a boiling hot water bath. 1. Each student will prepare 6 standards so that all 6 standards are run in one gel. Each student must also prepare his or her own unknown sample. You will prepare the ovalbumin sample from Lab 1*, and the molecular weight marker**. *Add 10 mg of ovalbumin to 1 mL of 1X PBS buffer. Continue with Step 2. o Note: it is likely that not all of your ovalbumin will dissolve. The use of a sonicator for 3 minutes may aid in dissolving your sample. Vortex immediately before removing 20 µL of your sample in Step 2. Do not do a “pop spin” prior to removing your 20 µL sample. Any undissolved material will go into solution upon heating in Laemmli sample buffer. **The marker will already be diluted. Use what is in the tube provided to you and continue with Step 4. Do not boil the marker. 2. Add 20 µL of each protein sample and standard into clearly labeled microcentrifuge tubes. Add 20 µL of Laemmli sample buffer and vortex briefly. 3. Place the tubes in a floating foam holder and heat the samples at 100 °C for 5 minutes in a water bath. Carefully remove the samples from the water bath and allow them to cool on your bench for 1 - 2 minutes prior to loading. 4. Load the samples as described below. PART 4: Sample Loading Practice loading (only if needed): to practice loading a sample into a well, load 5 µL of loading buffer into one of the wells that you will be using. Note: you can add your samples into the wells that contain loading buffer. 1. Load the samples into the wells with a pipette. You will load 20 µL of each sample (including the marker) per lane. 2. Load SLOWLY; do not “shoot” the sample out of the tip causing spillage and overflow from the individual well. Note: i) Slow but even loading allows the sample to settle evenly on the bottom of the well. ii) The pipette tip will not go into the well, but rather will gently rest against the plate at the top of the well. iii) You can use the same pipette tip to load all of your samples. Just thoroughly rinse with running buffer between sample loading. 3. Each gel has 10 lanes and should be loaded as shown below:
Lane:
_M_ 1
_U_ 2
_S1_ 3
_S2_ 4
_S3_ 5
_S4_ 6
_S5_ 7
_S6_ 8
_U_ 9
_O_ 10
Where: M = Molecular Weight Marker S = Protein Standard U = Unknown Sample (note – you have 2 lanes in order to place your unknown sample) Page 6
CHEMBIO2L03 LAB #2B
SDS-PAGE
O = Ovalbumin Sample •
This loading arrangement allows two students to use one gel. Each student will load the marker or the ovalbumin sample, their unknown, as well as 3 out of 6 standards.
PART 5: Gel Electrophoresis NOTE: You will need to wait until both pairs are ready before starting the gel. 1. Place the lid on the mini tank making sure to align the color coded banana plugs and jacks. A stop on the lid prevents incorrect orientation. The lid is secure when you hear a click. 2. Insert the electrical leads into a suitable power supply with the proper polarity. 3. Apply power to the Mini-PROTEAN® Tetra Cell and begin electrophoresis. Set the power supply for 200 volts (constant) and 40 minutes*. Be sure to note the time and to make sure that your samples are running into the gel. *NOTE: you will run your gel until the dye front reaches the bottom of the gel and is just about to run off. Gel running times may vary, so you may need to run your gel for longer or shorter than 40 minutes. PART 6: Gel Removal 1. After electrophoresis is complete, turn off the power supply and disconnect the electrical leads. 2. Remove the tank lid and carefully lift out the inner chamber assembly. Pour off the running buffer into the “USED RUNNING BUFFER” container provided. Note: Always pour off the buffer before opening the clamps to avoid spilling the buffer. 3. Open the green clamps of the clamping frame and remove the gel cassette sandwiches. 4. To open the cassette, align the arrow on the opening lever with the arrows marked on the cassette. Insert the lever between the cassette plates at all four locations and apply downward pressure to break the seal. Gently pull apart the two plates beginning at the top of the cassette. 5. Gently remove the gel by floating it off the plate by inverting the gel and plate into stain and gently agitate until the gel separates from the plate. Do this in a large dish which you will use for both staining and destaining your gel. Note: handle the gel with care as it tears easily. 6. Rinse the Mini-PROTEAN® Tetra Cell first with tap water and then with ddH2O after each use. Note: There is no need to use soap when rinsing the apparatus after use. The SDS in the running buffer is enough soap to clean the unit. TAKE GREAT CARE WHEN MANIPULATING THE MINI-PROTEAN® TETRA CELL ASSEMBLY SUCH THAT THE PLATINUM WIRE IN THE BOTTOM OF THE UNIT IS NOT DISTURBED. PART 7: Staining the Gel 1. Once you have placed the gel in staining solution, warm the gel for 30 seconds in the microwave to accelerate the staining process. Agitate gently every couple of minutes and allow the gel to stain for 25 minutes. Note: Coomassie Brilliant Blue stains the skin very effectively. If your skin becomes stained do not be alarmed - the stain is safe to humans and is even used intravenously to study cardiac efficiency/blood flow.
Page 7
CHEMBIO2L03 LAB #2B
SDS-PAGE
PART 8: Destaining the Gel 1. Carefully pour off the staining solution into the “USED STAINING SOLUTION” bottle. Gently hold your gel while pouring to ensure that you do not lose your gel. The staining solution can be reused many times. 2. Rinse with a very small amount of destaining solution. Pour off, and re-fill with destaining solution to cover the gel. 1. Warm the gel for 30 seconds in the microwave. Place three folded Kimwipes in the destaining solution with the gel to act as a sponge and remove the excess dye from the destaining solution. The Kimwipes should not be placed on top of the gel, but rather along the edge of the dish. Gently agitate the gel to facilitate destaining. 3. To aid in the destining process, change the destaining solution every 15 minutes and repeat step 3. 4. The gel should destain in an hour or less. Destaining is complete when the proteins appear as blue bands on a relatively transparent gel background.
PART 9: Photograph your Gel Once your gel has destained enough to visualize the protein bands consult your TA to obtain a picture of your gel. For best results, the gel should take up the entire field of view of the camera. Make sure to note the number of your gel so that you can identify it later. The digital picture of your gel will be posted on A2L so that you may print it for analysis. Make sure you have the correct gel. After you have taken a picture, dispose of your gel in the bin provided in the fume hood.
ANALYSIS AND DISCUSSION 1. For each protein band in the marker lane measure the distance from the top of the gel to the center of the band of interest (in mm). Also measure the distance from the top of the gel to the center of the dye front (in mm) in the same lane. If the dye front is not visible, you may use the smallest marker protein as the “front”. 2. Calculate the Rf value for each marker protein band. NOTE: the three largest marker proteins may not be resolved on your gel. 3. Use the Rf values for the marker proteins to construct a calibration plot of displacement versus log molecular weight. 4. Using the equation of the line from your calibration plot determine the molecular weights for the 6 protein standards, the bands in your unknown sample, and the ovalbumin. This should go into a table along with the identification (i.e. protein name) and the corresponding molecular weights for total clarity of presentation. The Rf values, MW’scalculated, MW’sknown, and the ID’s for the standards, unknowns and ovalbumin should be included in your table.
Page 8
CHEMBIO2L03 LAB #2B
•
•
SDS-PAGE
How does the efficiency of separation change as the molecular weight of the proteins increase? Why? What conditions would you alter to obtain better separation of high molecular weight proteins? Compare the known molecular weights of the standards with those obtained from your calibration plot. How much do the results deviate? What is the significance of this when determining the molecular weight of an unknown protein?
•
Compare molecular weight determination by SDS-PAGE and SEC. Discuss what method is more accurate and why.
•
Comment on the purity of the ovalbumin sample. Should the sample be pure? Explain.
REFERENCES NOTE: technical bulletins are not appropriate references for your lab reports or weekly summaries. 1. Acrylamide Polymerization – A Practical Approach. Bio-Rad Bul...
Similar Free PDFs

Dry Lab 2b - Chem 1310 2B dry lab
- 13 Pages

Lab 2B - Diskpart Exercise
- 11 Pages

Quiz #2B - Quiz #2B
- 1 Pages

2B Lab Data for lab 2
- 6 Pages

Unit 2B - Assignment unit 2B
- 9 Pages

Lab Report Chemical Composition
- 3 Pages

Chemical Kinetics - lab report
- 4 Pages

Chemical Kinetic Lab Report
- 2 Pages

Chemical Bonding Lab
- 7 Pages
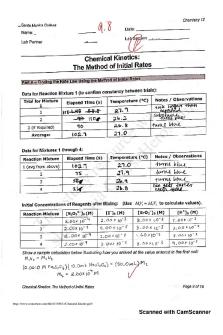
Chemical Kinetics lab
- 9 Pages

2b or Not 2b Discussion
- 1 Pages

Pea plant lab - Biology lab
- 6 Pages

Biology DNA Lab lab answers
- 18 Pages
Popular Institutions
- Tinajero National High School - Annex
- Politeknik Caltex Riau
- Yokohama City University
- SGT University
- University of Al-Qadisiyah
- Divine Word College of Vigan
- Techniek College Rotterdam
- Universidade de Santiago
- Universiti Teknologi MARA Cawangan Johor Kampus Pasir Gudang
- Poltekkes Kemenkes Yogyakarta
- Baguio City National High School
- Colegio san marcos
- preparatoria uno
- Centro de Bachillerato Tecnológico Industrial y de Servicios No. 107
- Dalian Maritime University
- Quang Trung Secondary School
- Colegio Tecnológico en Informática
- Corporación Regional de Educación Superior
- Grupo CEDVA
- Dar Al Uloom University
- Centro de Estudios Preuniversitarios de la Universidad Nacional de Ingeniería
- 上智大学
- Aakash International School, Nuna Majara
- San Felipe Neri Catholic School
- Kang Chiao International School - New Taipei City
- Misamis Occidental National High School
- Institución Educativa Escuela Normal Juan Ladrilleros
- Kolehiyo ng Pantukan
- Batanes State College
- Instituto Continental
- Sekolah Menengah Kejuruan Kesehatan Kaltara (Tarakan)
- Colegio de La Inmaculada Concepcion - Cebu


