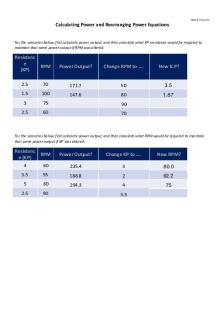
Hofmann Rearrangement PDF

| Title | Hofmann Rearrangement |
|---|---|
| Author | Sinead Goodall |
| Course | Further Organic and Biological Chemsitry |
| Institution | Cardiff University |
| Pages | 17 |
| File Size | 2 MB |
| File Type | |
| Total Downloads | 87 |
| Total Views | 119 |
Summary
...
Description
CHAPTER13 HofmannRearrangement BY, G.DEEPA
TheHofmannRearrangement
Treatment of amides with bromine in basic solution gives an amine with loss of the carbonyl carbon. O RCNH2
Br2 HO–
RNH2
+
CO32–
Examples O (CH3)3CCH2CNH2
Br2, NaOH
(CH3)3CCH2NH2
H2 O O
(94%)
Br2, KOH NH2
CNH2 H2 O Br
Br (87%)
MechanismoftheHofmannRearrangement
O RCNH2
O RCNHBr
RNCO
RNH2
•TheHofmannrearrangementinvolves6steps in3stages. • 1.formationofanN‐bromoamide(2steps) • 2.conversionoftheN‐bromoamidetoan isocyanate(2steps) • 3.hydrolysisoftheisocyanate(2steps)
Step1 ••
O •• – •• •O •
RC •N •
H
••
H
H
Step1 •• – • O• • •
•• • •
O •
H
RC •N •
H
H
•• •• O
H
H
Step2 •• – • O• • •
RC •N •
H
•• • Br • ••
••
Br •• ••
Step2 •• – • O• •
•• O ••
RC •N •
H
RC ••
Br •• ••
•• – • Br • • • ••
• •
H
••
Br •• ••
Step3 ••
O •• RC •N •
••
Br •• ••
H – •• •• O ••
H
Step3 •• – •O• • •
•• • •
R ••
Br •• ••
C •N •
••
Br •• ••
H – •• •• O ••
H
•• O ••
H
Step4 –
•• O ••
C R
•• • •O
R
N ••
r •• •• – • Br • • • ••
Steps5and6 •• •O •
•• O ••
H2 O
C R
C
N ••
R HO–
R
••
NH2
+
CO32–
N •• H
•• – O •• ••
MechanismofDiazonium saltformation H H
O
N
O
+
H
+
H
+
O
N
O
H +
+
O
N
H 2O
O
H
+
N
O
nitrosonium ion H +
R
NH
2
+
+
N
R
O
N
N
O
H N-nitrosoammonium ion H +
R
N
N
O
+
H 2O
R
N
N
+ H 3O
O
H N-nitrosamine
H
R
N
N
R
O
N
N
OH
tautomerization H
a diazenol
R
N
N
R
N
N
+
OH
+
H
+
+
R
N
H
O H
+
H R
O
N
N
N
H a diazonium ion
+
H 2O
+
Basicityofamines The greater the availability of the lone pair electrons on nitrogen, the greater the base. In the old days, pKb was a measure of base strength. Kb = [RNH3+] [OH-] / RNH2
pKb = - log Kb
The stronger the base the lower the pKb EFFECTS ON AMINE BASICITY 1. INDUCTIVE EFFECT - ALKYL SUBSTITUTION METHYL GROUP INCREASES ELECTRON DENSITY ON N
3.36
3.28
2 METHYLS ARE BETTER THAN ONE
WATCH OUT THREE METHYL GROUPS DECREASES BASICITY pKb = 4.26 - Steric inhibition of solvation of HOH with the NH+ of the R3NH+ cation.
The greater the availability of the lone pair electrons on nitrogen, the greater the base. In the old days, pKb was a measure of base strength. Kb = [RNH3+] [OH-] / RNH2
pKb = - log Kb
The stronger the base the lower the pKb EFFECTS ON AMINE BASICITY 1. INDUCTIVE EFFECT - ALKYL SUBSTITUTION METHYL GROUP INCREASES ELECTRON DENSITY ON N
3.36
3.28
2 METHYLS ARE BETTER THAN ONE
WATCH OUT THREE METHYL GROUPS DECREASES BASICITY pKb = 4.26 - Steric inhibition of solvation of HOH with the NH+ of the R3NH+ cation.
Reimer‐Tiemann ReactionMechanism
THANKYOU...
Similar Free PDFs

Hofmann Rearrangement
- 17 Pages

Skript Baurecht BW (Hofmann)
- 24 Pages

The Pinacol Rearrangement
- 6 Pages

Lab Report 9- pinacol rearrangement
- 15 Pages
Popular Institutions
- Tinajero National High School - Annex
- Politeknik Caltex Riau
- Yokohama City University
- SGT University
- University of Al-Qadisiyah
- Divine Word College of Vigan
- Techniek College Rotterdam
- Universidade de Santiago
- Universiti Teknologi MARA Cawangan Johor Kampus Pasir Gudang
- Poltekkes Kemenkes Yogyakarta
- Baguio City National High School
- Colegio san marcos
- preparatoria uno
- Centro de Bachillerato Tecnológico Industrial y de Servicios No. 107
- Dalian Maritime University
- Quang Trung Secondary School
- Colegio Tecnológico en Informática
- Corporación Regional de Educación Superior
- Grupo CEDVA
- Dar Al Uloom University
- Centro de Estudios Preuniversitarios de la Universidad Nacional de Ingeniería
- 上智大学
- Aakash International School, Nuna Majara
- San Felipe Neri Catholic School
- Kang Chiao International School - New Taipei City
- Misamis Occidental National High School
- Institución Educativa Escuela Normal Juan Ladrilleros
- Kolehiyo ng Pantukan
- Batanes State College
- Instituto Continental
- Sekolah Menengah Kejuruan Kesehatan Kaltara (Tarakan)
- Colegio de La Inmaculada Concepcion - Cebu