HW 1 - Mandatory Assignment - Organic Chemistry 1 - Spring Semester 2017 PDF
| Title | HW 1 - Mandatory Assignment - Organic Chemistry 1 - Spring Semester 2017 |
|---|---|
| Course | Organic Chemistry I |
| Institution | Georgia State University |
| Pages | 4 |
| File Size | 215.6 KB |
| File Type | |
| Total Downloads | 10 |
| Total Views | 128 |
Summary
Mandatory Assignment Organic Chemistry 1 Spring Semester 2017...
Description
Homework #1 Organic Chemistry
Name
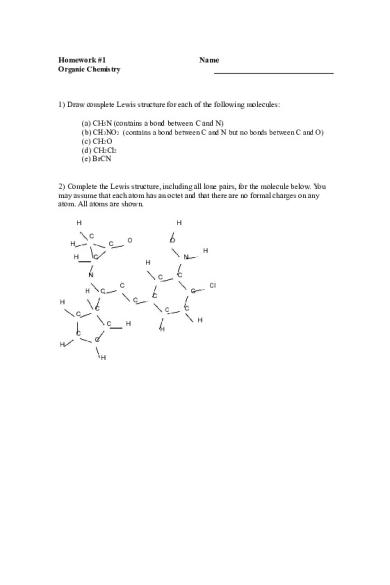
1) Draw complete Lewis structure for each of the following molecules: (a) CH5N (contains a bond between C and N) (b) CH3NO2 (contains a bond between C and N but no bonds between C and O) (c) CH2O (d) CH2Cl2 (e) BrCN
2) Complete the Lewis structure, including all lone pairs, for the molecule below. You may assume that each atom has an octet and that there are no formal charges on any atom. All atoms are shown. H
H C
H
O
C
O H
H
C
N H
N
C
C
Cl
C H
C C
H
C
C
C
C
C C
H
H H
C C H H
C
3) Determine the formal charge of every atom in every species in the sequence of reaction steps below. For any nonzero formal charge, write the formal charge next to the atom. If the formal charge on an atom is zero, write nothing next to the atom. NH
NH2
HO
C
H O
C
3
H3C
2
H3C
CH3
CH3
NH2
NH2
H O
C
2
H3C
C
CH3
H3C
CH3
OH2 NH2 NH2 C
HO
H2 O
H3C
C
CH3
3
H3C
OH2
CH3
OH NH3
NH2
H3O
C H3C
HO
C H3C
CH3
2
CH3
OH
OH NH3
CH3
H3C
C
NH3
C H3C
CH3 OH
OH
CH3
H3C C OH
NH3
CH3
H3C C O
NH4
4) For each species below, (i) Draw all resonance contributors and (ii) draw its resonance hybrid. Make sure to use the curved arrows on each structure to indicate how you get to the next resonance contributor. (Note: If all lone pairs are not shown, you should draw them in) H C
CH HC CH HC CH
C
H
a)
H C
CH HC CH HC CH
C
H
b)
O H C O
N C H
c)
O
H C C
O H 3C
d)
C C H
CH2
5) For each of the following molecular species, label each non-hydrogen atom with its electronic geometry—either linear, trigonal planar, or tetrahedral.
N
a)
HN
O
b)
2
6) Draw the complete Lewis structure for the following line structure, including all carbons, hydrogens and lone pairs. N
7) The dash-wedge notation of D-glucose is shown below. Draw its dash-wedge notation after each of the indicated rotations has taken place. (a) 180
OHOH
o
H OH O
OH
OH
D-glucose (b) OH
OH
o
180
H OH O
OH
OH
D-glucose...
Similar Free PDFs

Organic Chemistry HW 11
- 2 Pages

Clayden Organic Chemistry (1)
- 1,265 Pages

Organic Chemistry Lab 1
- 5 Pages

Organic Chemistry Lab 1
- 14 Pages

Organic Chemistry 1 2423
- 3 Pages

Essay 1 - Mandatory Assignment
- 5 Pages

Assignment 3 - organic chemistry
- 7 Pages

Hw 06 spring 2017 questions
- 1 Pages

Organic Chemistry II - QUIZ 1
- 2 Pages

Organic Chemistry Lab Report #1
- 8 Pages
Popular Institutions
- Tinajero National High School - Annex
- Politeknik Caltex Riau
- Yokohama City University
- SGT University
- University of Al-Qadisiyah
- Divine Word College of Vigan
- Techniek College Rotterdam
- Universidade de Santiago
- Universiti Teknologi MARA Cawangan Johor Kampus Pasir Gudang
- Poltekkes Kemenkes Yogyakarta
- Baguio City National High School
- Colegio san marcos
- preparatoria uno
- Centro de Bachillerato Tecnológico Industrial y de Servicios No. 107
- Dalian Maritime University
- Quang Trung Secondary School
- Colegio Tecnológico en Informática
- Corporación Regional de Educación Superior
- Grupo CEDVA
- Dar Al Uloom University
- Centro de Estudios Preuniversitarios de la Universidad Nacional de Ingeniería
- 上智大学
- Aakash International School, Nuna Majara
- San Felipe Neri Catholic School
- Kang Chiao International School - New Taipei City
- Misamis Occidental National High School
- Institución Educativa Escuela Normal Juan Ladrilleros
- Kolehiyo ng Pantukan
- Batanes State College
- Instituto Continental
- Sekolah Menengah Kejuruan Kesehatan Kaltara (Tarakan)
- Colegio de La Inmaculada Concepcion - Cebu





