[MCQ\'s] Renewable Energy System -module 6 PDF
![[MCQ\'s] Renewable Energy System -module 6](https://pdfedu.com/img/crop/300x450/0k36qlyzn4rd.jpg)
| Title | [MCQ\'s] Renewable Energy System -module 6 |
|---|---|
| Author | vaibhav bhardwaj |
| Course | Reneable energy resources |
| Institution | APJ Abdul Kalam Technological University |
| Pages | 7 |
| File Size | 127.4 KB |
| File Type | |
| Total Downloads | 34 |
| Total Views | 139 |
Summary
Mcqs...
Description
Use Your Vacations to Upskill yourself -> Explore the Power of Python and ML Click here!!!
Get Latest Exam Updates, Free Study materials and Tips Your Name Your Branch Year Of Engineering
[MCQ’s] Renewable Energy System Module 1 Module 2 Module 3 Module 4 Module 5 Module 6
Module 6
1. Which of the following supplies maximum amount of hydrogen gas? a) Natural gas
b) Anaerobic Digestion c) Wastewater treatment d) Electrolysis Answer: a 2. In terms of green house gas emissions, how good or bad is hydrogen fuel? a) Major contributor of greenhouse gas emissions b) Zero-emission fuel c) Lowest contributor of greenhouse gas emissions d) Hydrogen cannot be used as fuel Answer: b 3. Which of the following use hydrogen as fuel? a) Fossil fuels b) Anerobic digestion c) Fuel cells d) Cooking Answer: c 4. Which of the following is the most popular application of hydrogen fuel cell? a) Fuel cell vehicles b) Fuel cell energy power plants c) Fuel cells stand-alone power supplies d) Fuel cells spacecraft Answer: d 5. How is hydrogen gas produced from fossil fuels? a) Partial oxidation of methane b) Electrolysis c) Evaporation d) Biomass gasication Answer: a 6. What is the major drawback of steam-methane reforming technique to produce hydrogen?
a) Capital intensive b) Releases greenhouse gases into atmosphere c) A niche technology d) Poor eciency Answer: b 7. How does electrolysis produce hydrogen? a) By running electricity to combine hydrogen and water b) By separating water into hydrogen and oxygen and generating electricity c) By passing electricity into water to separate it into hydrogen and oxygen d) By passing electricity into water to evaporate it into hydrogen Answer: c 8. Why is hydrogen hazardous as fuel? a) Because of high ignition and low combustion energy b) Because of high ignition and high combustion energy c) Because low ignition and low combustion energy d) Because of low ignition and high combustion energy Answer: d 9. Traditionally, why is steam methane reforming preferred over electrolysis? a) Because electrolysis requires electricity b) Because electrolysis has lower production eciency c) Because steam methane reforming produces greenhouse gases d) Because electrolysis produces greenhouse gases Answer: a 10. What is the main problem in using hydrogen as fuel for vehicles? a) Capital intensive b) Storage c) Fuel cell technology is not well established d) Cars will become heavy Answer: b
11. What is a fuel cell? a) Converts heat energy to chemical energy b) Converts heat energy to electrical energy c) Converts chemical energy to electrical energy d) Converts kinetic energy to heat energy Answer: c 12. How does hydrogen fuel cell work? a) Membrane → hydrogen ions → electric current and recombination with oxygen b) Electric current and recombination with oxygen → hydrogen ions → membrane c) Hydrogen ions → membrane → electric current and recombination with oxygen d) Recombination with oxygen → electric current → membrane → hydrogen ions Answer: d 13. What does hydrogen fuel cell emit? a) Water b) Steam c) Greenhouse gas d) Methane Answer: a 14. Fuel cell vehicle is sourced by a battery. a) True b) False Answer: b 15. High pressure containers are used to store hydrogen. a) True b) False Answer: a 16. By what means can hydrogen be stored? a) Physically and chemically b) As atoms
c) As ions d) As fuel cells Answer: a 17. How is hydrogen stored physically? a) As atoms b) By compressing hydrogen gas c) In the form of hydrides d) In the form of water Answer: b 18. How is hydrogen stored chemically? a) As ions b) By compressing hydrogen gas c) In the form of hydrides d) In the form of ice Answer: c 19. Hydrogen molecules are dissociated either _________ or _________ to form hydrides. a) under sunlight or cold temperatures b) by substitution or electrolysis c) by electrolysis or heterolytically d) homolytically or heterolytically Answer: d 20. Cryogenic adsorption is a physical means to store hydrogen. a) True b) False Answer: a 21. What are the main components of a fuel cell? a) Anode, cathode, electrolyte b) Anode, cathode, membrane and electrolyte (including fuel) c) Anode, cathode d) Anode, cathode, electrolyte and connecting wires
Answer: b 22. What is hydrogen compressor do to hydrogen? a) Increases both pressure and volume b) Decreases both pressure and volume c) Increases pressure and decreases volume d) Decreases pressure and increases volume Answer: c 23. What is photocatalytic water splitting? a) Splitting of water using catalyst and electricity b) Splitting of water using electricity c) Combining hydrogen and oxygen to form water d) Splitting of water using light as catalyst Answer: d 24. Which of the following uses hydrogen as fuel? a) Vehicles b) AA battery c) AAA battery d) Power plants Answer: a 25. Eciency of water electrolysis is greater than the eciency of steam reforming. a) True b) False Answer: a
Prepare For Your Placements:https://lastmomenttuitions.com/courses/placement-preparation/
/ Youtube Channel:https://www.youtube.com/channel/UCGFNZxMqKLsqWERX_N2f08Q Follow For Latest Updates, Study Tips & More Content!
/lastmomenttuition /Last Moment Tuitions /lastmomentdost...
Similar Free PDFs

Nida who7 renewable energy
- 1 Pages

Lecture 5 AND 6 - Renewable Energy
- 16 Pages

Control System Mcqs
- 3 Pages

ENERGY FLUX. UNIT 6
- 6 Pages
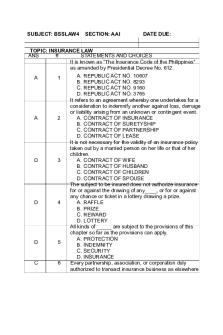
Topic 6 MCQs Business Law
- 94 Pages

Module 6
- 23 Pages
Popular Institutions
- Tinajero National High School - Annex
- Politeknik Caltex Riau
- Yokohama City University
- SGT University
- University of Al-Qadisiyah
- Divine Word College of Vigan
- Techniek College Rotterdam
- Universidade de Santiago
- Universiti Teknologi MARA Cawangan Johor Kampus Pasir Gudang
- Poltekkes Kemenkes Yogyakarta
- Baguio City National High School
- Colegio san marcos
- preparatoria uno
- Centro de Bachillerato Tecnológico Industrial y de Servicios No. 107
- Dalian Maritime University
- Quang Trung Secondary School
- Colegio Tecnológico en Informática
- Corporación Regional de Educación Superior
- Grupo CEDVA
- Dar Al Uloom University
- Centro de Estudios Preuniversitarios de la Universidad Nacional de Ingeniería
- 上智大学
- Aakash International School, Nuna Majara
- San Felipe Neri Catholic School
- Kang Chiao International School - New Taipei City
- Misamis Occidental National High School
- Institución Educativa Escuela Normal Juan Ladrilleros
- Kolehiyo ng Pantukan
- Batanes State College
- Instituto Continental
- Sekolah Menengah Kejuruan Kesehatan Kaltara (Tarakan)
- Colegio de La Inmaculada Concepcion - Cebu
![[MCQ\'s] Renewable Energy System -module 6](https://pdfedu.com/img/crop/172x258/0k36qlyzn4rd.jpg)








