Modeling of Chemical Kinetics and Reactor Design - A. Kayode Coker.pdf PDF

| Title | Modeling of Chemical Kinetics and Reactor Design - A. Kayode Coker.pdf |
|---|---|
| Author | Leonel Gomez |
| Pages | 1,126 |
| File Size | 14.2 MB |
| File Type | |
| Total Downloads | 11 |
| Total Views | 227 |
Summary
Modeling of Chemical Kinetics and Reactor Design Modeling of Chemical Kinetics and Reactor Design A. Kayode Coker, Ph.D. Lecturerer and Consultant, AKC Technology Boston Oxford Johannesburg Melbourne New Delhi Singapore Dedication To my wife Victoria and the boys Akin and Ebun, love and thanks. Mod...
Description
Modeling of Chemical Kinetics and Reactor Design
Modeling of Chemical Kinetics and Reactor Design A. Kayode Coker, Ph.D. Lecturerer and Consultant, AKC Technology
Boston Oxford Johannesburg Melbourne New Delhi Singapore
Dedication To my wife Victoria and the boys Akin and Ebun, love and thanks.
Modeling of CHEMICAL KINETICS AND REACTOR DESIGN
Copyright © 2001 by Gulf Publishing Company, Houston, Texas. All rights reserved. Printed in the United States of America. This book, or parts thereof, may not be reproduced in any form without permission of the publisher. Gulf Publishing Company Book Division P.O. Box 2608, Houston, Texas 77252-2608 Library of Congress Cataloging-in-Publication Data (To come) ISBN 0-88415-481-5 Printed in the United States of America. Printed on acid-free paper (∞). iv
Acknowledgments I wish to express my gratitude to the following for giving their time in proofreading various sections of the text: Drs. A. A. Adesina, L. M. Rose, C. J. Mumford, and J. D. Jenkins. I am indebted to Emeritus Professor Octave Levenspiel for his encouragement and advice in some chapters of the text, and to Drs. Waldram and Singh for their comments, suggestions on safety in reaction engineering, and the inclusion of HEL safety photographs in the text. I would also like to thank Mr. Ed Steve for his comments and suggestions on scale-up of reactors, and Mr. Joseph Rivera for some of the figures in the text. I wish to express my gratitude to Drs. A. Bakker, J. B. Fasano, and V. V. Ranade for contributing to Chapter 10 (Computational Fluid Dynamics and Computational Fluid Mixing). I would like to acknowl-edge the following companies for the use of their materials: Arthur D. Little, HEL, M. W. Kellogg Ltd., Stone & Webster, Fauske & Associates, Inc., Simulation Sciences Inc., Chemineer, PROCEDE, and Absoft Corporation. I would like to express my gratitude to the following institutions for permission to reproduce their materials: Institution of Chemical Engineers (U.K.), the American Institute of Chemical Engineers and Chemical Engineering—a publication of Chemical Week Associates. I am also indebted to those whose work was drawn. I thank Dr. E. L. Smith for his comments and suggestions during the preparation of some of the chapters in the text and wish him a happy retirement. It has been a pleasure to have learned so much from him during his tenure at Aston University. Sincere gratitude to Tim Calk of Gulf Publishing Company for his direction and editing of the book, to Danette DeCristofaro and Jerry Hayes of ExecuStaff for their excellent production of the book, to Mr. Phil Carmical and Ms. Jennifer Plumley of Butterworth-Heinemann for the production of the CD-ROM. v
My thanks to Ahmed Mutawa of Saudi Aramco Shell Refinery (SASREF), an excellent student in the short course program for developing conversion table software for the book. In gratitude to the Almighty father, the Omnipotence, Omniscience, and Omnipresence. A. Kayode Coker, Ph.D.
Nippon Petroleum FCC Unit—Japan. (Courtesy M. W. Kellogg Ltd.) vi
Contents Preface ................................................................................................. xiii Introduction ........................................................................................ xvii CHAPTER ONE Reaction Mechanisms and Rate Expressions ................................ 11 Introduction 1 Typical Reaction Mechanisms 5 Reaction Mechanisms 8 Elementary and Non-Elementary Reactions 9 Types of Intermediate 10 The Arrhenius Equation and the Collision Theory 12 Transition State Theory 15 Chain Reactions 16 Catalytic Reactions 21 Guidelines to Formulating Reaction Mechanism 32 Testing Kinetic Models 34 Chain Length 37 References 58 CHAPTER TWO Thermodynamics of Chemical Reactions ....................................... 59 Introduction 59 Chemical Equilibrium 60 Criteria for Equilibrium 63 Reaction Equilibrium 64 Ideal Gas Mixtures 65 Real Gases—Ideal Gaseous Solutions 65 Real Gases 67 Liquid State 69 Determining the Fugacity and the Fugacity Coefficient 70 Partial Molar Quantities 72 Effect of Temperature on the Equilibrium Constant 74 vii
Heats of Reaction 75 Heat Capacities of Gases 80 Heats of Formation 80 References 93 Appendix 94 CHAPTER THREE Reaction Rate Expression ................................................................ 109 Introduction 109 Reaction Rate Equation 110 Reaction Orders 114 Determining the Order of Reactions 116 Empirical Rate Equations of the nth Order 129 Method of Half-Life t1/2 130 Parallel Reactions 134 Homogeneous Catalyzed Reactions 137 Autocatalytic Reactions 138 Irreversible Reactions in Series 140 First Order Reversible Reactions 146 Second Order Reversible Reactions 150 General Reversible Reactions 151 Simultaneous Irreversible Side Reaction 152 Pseudo-Order Reaction 154 Practical Measurements of Reaction Rates 155 Regression Analysis 171 Weighted Least Squares Analysis 173 Problems and Errors in Fitting Rate Models 175 References 216 CHAPTER FOUR Industrial and Laboratory Reactors ............................................. 218 Introduction 218 Batch Isothermal Perfectly Stirred Reactor 220 Semi-Batch Reactors 222 Continuous Flow Isothermal Perfectly Stirred Tank Reactor 226 Continuous Isothermal Plug Flow Tubular Reactor 227 Continuous Multiphase Reactors 230 Fluidized Bed System 232 Fluid Catalytic Cracking (FCC) Unit 234 Deep Catalytic Cracking Unit 235 Determining Laboratory Reactors 243 Guidelines for Selecting Batch Processes 254 viii
Guidelines for Selecting Batch Processes 254 References 259 CHAPTER FIVE Introduction to Reactor Design Fundamentals for Ideal Systems ............................................................................... 260 Introduction 260 A General Approach 262 Ideal Isothermal Reactors 264 Numerical Methods for Reactor Systems Design 279 Reversible Series Reactions 287 The Semibatch Reactor 306 Continuous Flow Stirred Tank Reactor (CFSTR) 312 Multi-Stage Continuous Flow Stirred Tank Reactor 327 Equal Size CFSTR In Series 334 Space Time (ST) and Space Velocity (SV) 349 Fractional Conversion, Yield, and Selectivity in Reactors 351 Relationship Between Conversion, Selectivity, and Yield 353 Plug Flow Reactor 362 Heterogeneous Tubular Reactor 371 Design Equation for Systems of Variable Density 372 Design Equations for Heterogeneous Reactions 375 Comparison of Ideal Reactors 387 CFSTR and Plug Flow Systems 396 Dynamic Behavior of Ideal Systems 400 Flow Recycle Reactor 410 References 423 CHAPTER SIX Non-Isothermal Reactors ................................................................. 424 Introduction 424 Operating Temperature, Reaction Types, and Temperature 425 Effect of Operating Parameters on Equilibrium Conversion 429 Energy Balance and Heat of Reaction 429 Energy Transferred between the System and Surroundings 434 Batch Reactor 457 Plug Flow Reactor 472 Autothermal Reactors 477 Conversion in Ammonia Synthesis 478 Two-Dimensional Tubular (Plug Flow) Reactor 492 Pressure Drop (∆P) in Tubular (Plug Flow) Reactors 494 Thermal Behaviors in Flow Systems 500 ix
Exothermic Reactions in CFSTRs 504 Thermal Behavior of a Tubular Flow Reactor 507 Variable Coolant Temperature in a CFSTR 515 Optimal Design of Non-Isothermal Reactors 518 Mimimum Reactor Volume at the Optimum Temperature Progression (OTP) of a Single CFSTR with a Reversible Exothermic Reaction 543 Optimum Reactor Size 546 References 551 CHAPTER SEVEN Fluid Mixing in Reactors ................................................................ 552 Introduction 552 Mixing and Agitation of Fluids 553 Similarity 570 Mixing Time Correlation 578 Scale-up of Mixing Systems 584 Static Mixers 597 Heat Transfer in Agitated Vessels 615 Liquid-Solid Agitation 634 Batch Heating and Cooling of Fluids 636 Design of Mixing Systems 656 References 659 CHAPTER EIGHT Residence Time Distributions in Flow Reactors ......................... 663 Introduction 663 The Residence Time Distribution Functions and their Relationships 664 Determining RTD from Experimental Tracer Curves 680 Analysis of RTD from Pulse Input 688 Residence Time Distribution for a Laminar Flow Tubular Reactor 708 E- and F-Curves for a Series of Stirred Tank Reactors 713 RTD Functions for CSTRs Where N Is Not an Integer 721 The Dispersion Model 723 Comparison of Tank In Series (TIS) and Dispersion Plug Flow (DPF) Models 746 Residence Time Distribution in a Static Mixer 747 Glossary 756 References 760 x
CHAPTER NINE Models for Non-Ideal Systems ........................................................ 762 Introduction 762 Basics of Non-Ideal Flow 762 Segregated Flow Model 764 Complete Segregation Model with Side Exits 770 Maximum Mixedness Model (MMM) 772 Effect of Micromixing on Conversion 774 References 782 CHAPTER TEN Application of Computational Fluid Dynamics and Computational Fluid Mixing in Reactors ............................ 783 Introduction 783 Theory and Fluid Flow Equations 786 Turbulence on Time-Averaged Navier-Stokes Equations 792 Time-Dependent Turbulent Mixing and Chemical Reaction in Stirred Tanks 794 References and Recommended Reading 810 Nomenclature 810 Improve Reactors Via Computational Fluid Dynamics 811 References 828 CHAPTER ELEVEN Biochemical Reaction ....................................................................... 830 Introduction 830 Kinetics of Enzyme-Catalyzed Reactions 831 Models of Enzyme Kinetics 834 Enzyme Kinetics in the Presence of an Inhibitor 851 Fermentation 853 Design of Biological Reactors 855 Vessel Design and Aspect Ratio 857 Types of Operation 863 Cell Growth 863 Modeling Biological Reactors 868 General Model for a Single Vessel 872 The Chemostat 876 Batch Fermenter 884 Fed-Batch Reactor 887 Scale-up of Bioreactors 889 Nomenclature 898 xi
Glossary 899 References 908 CHAPTER TWELVE Safety in Chemical Reaction Engineering .................................... 910 Introduction 910 Hazard Evaluation in the Chemical Process Industries 911 Hazard Assessment Procedures 916 Thermal Runaway Chemical Reaction Hazards 919 The φ-Factor 920 Onset Temperature 923 Test Instruments 926 Two-Phase Flow Relief Sizing for Runaway Reaction 950 Vent Sizing Methods 963 Discharge System 973 Inherently Safe Plants in Reactor Systems 984 Hazard and Operability Studies (HAZOP) 991 Glossary 1010 References 1018 Appendix 1022 CHAPTER THIRTEEN Scale-Up in Reactor Design .......................................................... 1034 Introduction 1034 Development and Scale-Up of Reactors 1036 Similarity Criteria 1037 Scale-Up in Relation to Various Factors 1037 Heat Effect 1038 Coefficients of Process Stability 1039 Dimensional Analysis and Scale-Up Equations 1040 Mathematical Modeling 1044 Scale-Up of a Batch Reactor 1047 Heat Transfer Model 1057 Jacket Zoning of a Batch Reactor 1065 The Outlet Temperature of a Scaled-Up Batch System 1070 Aspect Ratio (R) in Jacket Zoning and Scale-Up of a Batch Reactor 1074 Nomenclature 1079 References 1080 Nomenclature ................................................................................... 1082 Index .................................................................................................. 1089 About the Author ............................................................................ 1096 xii
Preface SCOPE This valuable reference volume conveys a basic understanding of chemical reactor design methodologies that incorporate both scale-up and hazard analysis. It shows readers how to select the best reactor for any particular chemical reaction, how to estimate its size, and how to obtain the best operating conditions. An understanding of chemical reaction kinetics and the design of chemical reactors is very important to the chemist and the chemical engineer. Engineers share interests in fluid mechanics and transport phenomena, while the chemist deals with the kinetics and mechanisms of reactions. The chemical engineer combines the knowledge of these subjects for the better understanding, design, and control of the reactor. The recent accidents that have occurred in the chemical process industries with inherent fatalities and environmental pollution have imposed greater demands on chemical engineers. Consequently, chemical reactor design methodologies must incorporate both control and hazard analysis. However, the design of chemical reactors is still essential for its proper sizing, and is included in various types of process simulators. In an industrial problem, it is essential to select the best type of reactor for any particular chemical reaction. Additionally, it is necessary to estimate its size and determine the best operating conditions. The chemical engineer confronted with the design of various reactor types often depends on the scale of operation and the kinetics. Many excellent texts have appeared over the years on chemical reactor design. However, these texts often lack sections on scale-up, biochemical reactor design, hazard analysis, and safety in reactor design methodology. The purpose of this book is to provide the basic theory and design and, sometimes, computer programs (Microsoft Excel spreadsheet and software) for solving tedious problems. This speeds up the work of both chemists and engineers in readily arriving at a solution. The following highlights some of the subjects that are covered in this text. xiii
MIXING An important unit operation in chemical reaction engineering, mixing, finds application in petrochemicals, food processing, and biotechnology. There are various types of fluid mixing such as liquid with liquid, gas with liquid, or solids with liquid. The text covers micromixing and macromixing, tracer response and residence time distribution (RTD), heat transfer, mixing fundamentals, criteria for mixing, scale of segregation, intensity of segregation, types of impellers, dimensional analysis for liquid agitation systems, design and scale-up of mixing pilot plants, the use of computational fluid dynamics (CFD) in mixing, and heat transfer in agitated vessels. BIOCHEMICAL REACTION This is an essential topic for biochemists and biochemical engineers. Biochemical reactions involve both cellular and enzymatic processes, and the principal differences between biochemical and chemical reactions lie in the nature of the living systems. Biochemists and biochemical engineers can stabilize most organic substances in processes involving microorganisms. This chapter discusses the kinetics, modeling and simulation of biochemical reactions, types and scale-up of bioreactors. The chapter provides definitions and summary of biological characteristics. CHEMICAL REACTOR MODELING This involves knowledge of chemistry, by the factors distinguishing the micro-kinetics of chemical reactions and macro-kinetics used to describe the physical transport phenomena. The complexity of the chemical system and insufficient knowledge of the details requires that reactions are lumped, and kinetics expressed with the aid of empirical rate constants. Physical effects in chemical reactors are difficult to eliminate from the chemical rate processes. Non-uniformities in the velocity, and temperature profiles, with interphase, intraparticle heat, and mass transfer tend to distort the kinetic data. These make the analyses and scale-up of a reactor more difficult. Reaction rate data obtained from laboratory studies without a proper account of the physical effects can produce erroneous rate expressions. Here, chemical reactor flow models using mathematical expressions show how physical xiv
processes interact with chemical processes. The proposed model must represent the flow behavior of an actual reactor, which is realistic enough to give useful information for its design and analysis. The text reviews different reactor flow models. SAFETY IN CHEMICAL REACTION Equipment failures or operator errors often cause increases in process pressures beyond safe levels. A high increase in pressure may exceed the set pressure in pipelines and process vessels, resulting in equipment rupture and causing major releases of toxic or flammable chemicals. A proper control system or installation of relief systems can prevent excessive pressures from developing. The relief system consists of the relief device and the associated downstream process equipment (e.g., knock-out drum, scrubber, absorbers, and flares) that handles the discharged fluids. Many chemical reactions (e.g., polymerization, sulphonation, nitration) in the chemical process industry result in runaway reactions or two-phase flow. This occurs when an exothermic reaction occurs within a reactor. If cooling no longer exists due to a loss of cooling water supply or failure of a control system (e.g., a valve), then the reactor temperature will rise. As the temperature rises, the reaction rate increases, leading to an increase in heat generation. This mechanism results in a runaway reaction. The pressure within the reactor increases due to increased vapor pressure of the liquid components and gaseous decomposition products as a result of the high temperature. Runaway reactions can occur within minutes for large commercial reactors and have resulted in severe damage to a complete plant and loss of lives. This text examines runaway reactions and two-phase flow relief. SCALE-UP The chemical engineer is concerned with the industrial application of processes. This involves the chemical and microbiological conversion of material with the transport of mass, heat and momentum. These processes are scale-dependent (i.e., they may behave differently in small and large-scale systems) and include heterogeneous chemical reactions and most unit operations. The heterogeneous chemical reactions (liquid-liquid, liquid-gas, liquid-solid, gas-solid, solid-solid) generate or consume a considerable amount of heat. However, the course of xv
such chemical reactions can be similar on both small and large scales. This happens if the mass and heat transfer processes are identical and the chemistry is the same. Emphasis in this text is on dimensional analysis with respect to the following: • Continuous chemical reaction processes in a tubular reactor. • Influence of back mixing (macromixing) on the degree of conversion and in continuous chemical reaction operation. • Influence of micro mixing on selectivity in a continuous chemical reaction process. • Scale-up of a batch reactor AN INTEGRATING CASE STUDY—AMMONIA SYNTHESIS This book briefly reviews ammonia synthesis, its importance in the chemical process industry, and safety precautions. This case study is integrated into several chapters in the text. See the Introduction for further details. Additionally, solutions to problems are presented in the text and the accompanying CD contains computer programs (Microsoft Excel spreadsheet and software) for solving modeling problems using numerical methods. The CD also contains colored snapshots on computational fluid mixing in a reactor. Additionally, the CD contains the appendices and conversion table software. A. Kayode Coker, Ph.D.
xvi
Introduction Increased collaboration between chemical reaction engineering and chemistry disciplines in recent years has produced significant advances in kinetic and thermodynamic modeling of processes. Additionally, improvement in analytical chemistry techniques, the formulation of mathematical models, and the development of computational tools have led to a deeper understanding of complex chemical reaction kinetics, particularly in mixtures with large numbers of compounds. Activities in both academic and industrial research organizations have enabled these groups to review the state of the art and cooperate with the overall objectives of improving the safety, yields, and quality of the products. Also, the final commitment to the production of any chemical product often depends on its profitability and other economic factors. Chemical kinetics mainly relies on the rates of chemical reactions and how these depend on factors such as concentration and temperature. An understanding of chemical kinetics is important in providing essential evidence as to the mechanisms of chemical processes. Although important evidence...
Similar Free PDFs

Reactor Design
- 27 Pages

Topic 15 Chemical Kinetics
- 5 Pages
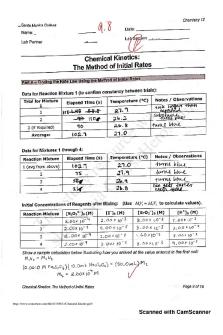
Chemical Kinetics lab
- 9 Pages

Chapter 14- Chemical Kinetics
- 3 Pages

Chemical Kinetics-DR. ASM
- 44 Pages

Chemical Kinetics - lab report
- 4 Pages

Isothermal Reactor Design
- 9 Pages

Lab 11 Chemical Kinetics Report
- 4 Pages

Chemical Kinetics - Lecture notes 1
- 23 Pages

Chemical Process Design and Simulation
- 418 Pages
Popular Institutions
- Tinajero National High School - Annex
- Politeknik Caltex Riau
- Yokohama City University
- SGT University
- University of Al-Qadisiyah
- Divine Word College of Vigan
- Techniek College Rotterdam
- Universidade de Santiago
- Universiti Teknologi MARA Cawangan Johor Kampus Pasir Gudang
- Poltekkes Kemenkes Yogyakarta
- Baguio City National High School
- Colegio san marcos
- preparatoria uno
- Centro de Bachillerato Tecnológico Industrial y de Servicios No. 107
- Dalian Maritime University
- Quang Trung Secondary School
- Colegio Tecnológico en Informática
- Corporación Regional de Educación Superior
- Grupo CEDVA
- Dar Al Uloom University
- Centro de Estudios Preuniversitarios de la Universidad Nacional de Ingeniería
- 上智大学
- Aakash International School, Nuna Majara
- San Felipe Neri Catholic School
- Kang Chiao International School - New Taipei City
- Misamis Occidental National High School
- Institución Educativa Escuela Normal Juan Ladrilleros
- Kolehiyo ng Pantukan
- Batanes State College
- Instituto Continental
- Sekolah Menengah Kejuruan Kesehatan Kaltara (Tarakan)
- Colegio de La Inmaculada Concepcion - Cebu





