P H Worksheet Solutions for ph scale and Poh scale for chemistry concepts PDF
| Title | P H Worksheet Solutions for ph scale and Poh scale for chemistry concepts |
|---|---|
| Author | Moksh Thakore |
| Course | Chemistry Concepts |
| Institution | St. Catherine University |
| Pages | 3 |
| File Size | 200.2 KB |
| File Type | |
| Total Downloads | 89 |
| Total Views | 127 |
Summary
P H Worksheet Solutions for ph scale and Poh scale for chemistry concepts. HHHhhsdaldkjs;ladkfhja lskdbfhlksdjhbfkjsadfncjhwodiunhwoeiufhwnofiusdhfinouhadnsuhfiouasdhfinoadsfchsiufghnisauhgcoiusndhgufhdsoiughcnfduhgoiusdfgucdnisfughoiudfhginovundfoughdfinounghoiudfhgkdjfhgkjdfhkljghsdlkjhglkjfdhglkj...
Description
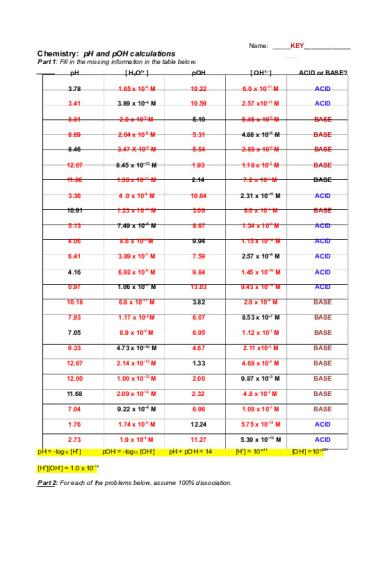
Name: _____KEY_____________
Chemistry: pH and pOH calculations Part 1: Fill in the missing information in the table below. pH
[ H3O1+ ]
pOH
[ OH1– ]
ACID or BASE?
3.78
1.65 x 10-4 M
10.22
6.0 x 10-11 M
ACID
3.41
3.89 x 10–4 M
10.59
2.57 x10-11 M
ACID
8.81
2.0 x 10-9 M
5.19
6.46 x 10-6 M
BASE
8.69
2.04 x 10-9 M
5.31
4.88 x 10–6 M
BASE
8.46
3.47 X 10-9 M
5.54
2.88 x 10-6 M
BASE
12.07
8.45 x 10–13 M
1.93
1.18 x 10-2 M
BASE
11.86
1.38 x 10-12 M
2.14
7.2 x 10-3 M
BASE
3.36
4 .0 x 10-4 M
10.64
2.31 x 10–11 M
ACID
10.91
1.23 x 10-11 M
3.09
8.0 x 10-4 M
BASE
5.13
7.49 x 10–6 M
8.87
1.34 x 10-9 M
ACID
4.06
8.6 x 10-5 M
9.94
1.15 x 10-10 M
ACID
6.41
3.89 x 10-7 M
7.59
2.57 x 10–8 M
ACID
4.16
6.92 x 10-5 M
9.84
1.45 x 10-10 M
ACID
0.97
1.06 x 10–1 M
13.03
9.43 x 10-14 M
ACID
10.18
6.6 x 10-11 M
3.82
2.0 x 10-4 M
BASE
7.93
1.17 x 10-8 M
6.07
8.53 x 10–7 M
BASE
7.05
8.9 x 10-8 M
6.95
1.12 x 10-7 M
BASE
9.33
4.73 x 10–10 M
4.67
2.11 x10-5 M
BASE
12.67
2.14 x 10-13 M
1.33
4.68 x 10-2 M
BASE
12.00
1.00 x 10-12 M
2.00
9.87 x 10–3 M
BASE
11.68
2.09 x 10-12 M
2.32
4.8 x 10-3 M
BASE
7.04
9.22 x 10–8 M
6.96
1.08 x 10-7 M
BASE
1.76
1.74 x 10-3 M
12.24
5.75 x 10-13 M
ACID
2.73
1.9 x 10-4 M
11.27
5.39 x 10–12 M
ACID
pH = -log10 [H+]
pOH = -log10 [OH-]
pH + pOH = 14
[H+][OH-] = 1.0 x 10-14 Part 2: For each of the problems below, assume 100% dissociation.
[H+] = 10-pH
[OH-] =10-pOH
1.
A.
Write the equation for the dissociation of hydrochloric acid.
HCl(aq) → H+(aq) + Cl (
B.
Find the pH of a 0.00476 M hydrochloric acid solution.
pH =l o g . 00476 10 0 pH =2. 32
2.
A.
Write the equation for the dissociation of sulfuric acid.
aq)+HSO4-( aq) H2SO4( aq) → H+( HSO4-( aq) → H+( aq)+SO42-( aq) OR
aq)+SO42-( aq) H2SO4( aq) → 2H+( B.
Find the pH of a solution that contains 3.25 g of H2SO4 dissolved in 2.75 liters of solution.
Fi r s t ,findt hec onc ent r at i o n:M =( mol / L) Mol=( 3. 25g/ 98g/ mol )=0. 0332mol M =( 0. 0332mol / 2. 75L)=0. 0121M [ H+]=2(0. 0121)M pH =l og100. 0242=1. 62 3.
A.
Write the equation for the dissociation of sodium hydroxide.
NaOH( aq) → Na+( aq)+OHB.
Find the pH of a 0.000841 M solution of sodium hydroxide.
0. 000841M NaOH → [ OH-]=0. 000841M pOH =l og10 [ OH-]=l og100. 000841=3. 08 pH =14–3. 08=10. 92
4.
A.
Write the equation for the dissociation of aluminum hydroxide. 3+ ( aq)+3OH-( aq) Al ( OH) aq) → Al 3(
B.
If the pH is 9.85, what is the concentration of the aluminum hydroxide solution?
pOH + pH =14 pOH = (14- pH) = (14-9.85) = 4.15 [OH-] = 10-pOH
5.
A.
Write the equation for the dissociation of calcium hydroxide.
Ca( OH) aq) →Ca 2( ( ) B.
2+
( aq)+2OH-
If the pH is 11.64 and you have 2.55 L of solution, how many grams of calcium hydroxide are in the solution?
pOH =( 14pH)=( 1411. 64)=2. 36 [ OH-]=10-pOH =10-2.36=4. 4x10-3M M =( mol / L) MolNaOH =( M xL)=( 4. 4x10-3mol / Lx2. 55L)=0. 0111 Mas sNaOH =( 0. 0111molx40g/ mol )=0. 445g...
Similar Free PDFs

pH scale discussion reply
- 1 Pages

Ph e poh - Ph e poh
- 13 Pages

Taller p H y pOH
- 4 Pages

Braden scale - tools for success
- 1 Pages

Measurement and Scale Lecture
- 2 Pages

H&PPsych - Template for H&P
- 15 Pages

Scale vineland
- 1 Pages

Time scale
- 4 Pages
Popular Institutions
- Tinajero National High School - Annex
- Politeknik Caltex Riau
- Yokohama City University
- SGT University
- University of Al-Qadisiyah
- Divine Word College of Vigan
- Techniek College Rotterdam
- Universidade de Santiago
- Universiti Teknologi MARA Cawangan Johor Kampus Pasir Gudang
- Poltekkes Kemenkes Yogyakarta
- Baguio City National High School
- Colegio san marcos
- preparatoria uno
- Centro de Bachillerato Tecnológico Industrial y de Servicios No. 107
- Dalian Maritime University
- Quang Trung Secondary School
- Colegio Tecnológico en Informática
- Corporación Regional de Educación Superior
- Grupo CEDVA
- Dar Al Uloom University
- Centro de Estudios Preuniversitarios de la Universidad Nacional de Ingeniería
- 上智大学
- Aakash International School, Nuna Majara
- San Felipe Neri Catholic School
- Kang Chiao International School - New Taipei City
- Misamis Occidental National High School
- Institución Educativa Escuela Normal Juan Ladrilleros
- Kolehiyo ng Pantukan
- Batanes State College
- Instituto Continental
- Sekolah Menengah Kejuruan Kesehatan Kaltara (Tarakan)
- Colegio de La Inmaculada Concepcion - Cebu







