Pharmacology 1 - Determination of the LD50 of Propoxur in American Nymph Cockroaches PDF

| Title | Pharmacology 1 - Determination of the LD50 of Propoxur in American Nymph Cockroaches |
|---|---|
| Course | Pharmacology 1 |
| Institution | University of Technology Sydney |
| Pages | 9 |
| File Size | 469.5 KB |
| File Type | |
| Total Downloads | 29 |
| Total Views | 123 |
Summary
LD50 Report...
Description
PHARMACOLOGY 1 - Determination of the LD50 of propoxur in American Nymph Cockroaches Introduction: The American cockroach, scientifically classified as Periplaneta americana, is reddish brown in colour with a yellow band just behind the head. It is the largest of the common household cockroaches in Australia, ranging from approximately 30-50mm in size (Robinson 2005). P. americana undergoes three stages in its life cycle: egg, nymph and adult (Rozendaal 1997). Female adults lay an egg capsule (ootheca), and once the egg hatches, the nymphal life cycle begins (Rozendaal 1997). Nymphs are smaller than adult cockroaches, and do not have any wings. Development from the nymph to adult may take several months to a year (Rozendaal 1997). The species commonly inhabits dark areas of food-storage facilities, and garbage disposal zones of grocery stores and restaurants. The American cockroach is omnivorous and its diet ranges quite broadly from decaying organic matter, to leather and starch in book bindings (Jacobs 2013). Due to this diverse diet, the American cockroach has proved to be quite a versatile pest, infesting indoor and outdoor environments. In addition, P. americana produce odorous secretions that alter food flavour and smell (Jacobs 2013). The species has also been linked to disease, since pathogens, including Escherichia coli and Salmonella, have been found on the limbs of American cockroaches (Kassiri et al. 2014). These disease-causing microorganisms are then deposited on the food and utensils the cockroach treads on, increasing the risk of transmission to humans. Therefore, control of these pests is vital for hygienic and health purposes. This is achieved through the use of insecticides. Carbamate insecticides are toxic to insects through their ability to inactivate acetylcholinesterase (AChE) (Fukuto 1990). This enzyme is responsible for the breakdown of the neurotransmitter, acetylcholine (ACh), by hydrolysing it into acetate and choline (Colovic et al. 2013). As such, an excess of ACh builds up and accumulates at the nerve terminals of synapses and neuromuscular junctions (King & Aaron 2015). This high concentration of ACh continues to stimulate the nerve/muscle, leading to constant contraction, exhaustion and eventually, respiratory failure (Fukuto 1990). Carbamates form an enzyme-inhibitor complex with acetylcholinesterase, and later, bind to the serine hydroxyl group of the enzyme (Fukuto 1990). This carbamylated serine is fairly resistant to hydrolytic cleavage, hence rendering the enzyme active site inhibited (Cannon 2007). However, the physiological activity of carbamates is reversible. Within a few hours, the carbamyl is split from AChE via hydrolysis, ensuring that the insecticide is not overly toxic to humans (Cannon 2007). The carbamate propoxur is the 1
insecticide utilised in this experiment. It is one of the main active ingredients found in Baygon® insecticides. Due to its reversible mode of action, moderate use as a household insecticide is relatively safe for humans. The LD50 of a drug is the lethal concentration (mg/kg) required to kill 50% of the population sample. It is a measure of the drug’s toxicity, and therefore, the concentrations that may be harmful to humans. Since propoxur is a toxin found in consumer products, it is important to determine its LD50. Additionally, in regards to pest control, the LD50 values of different insecticides may be compared to determine their efficacy. As such, this experiment was performed in order to determine the LD50 of propoxur on nymphal stage American cockroaches.
Method: The experiment was conducted according to the instructions listed in the Pharmacology 1 laboratory manual, found on pages 29-35. Changes to method: The 25 mL prepared solution of 1% w/v concentration was prepared with 0.2561g of 97.6% pure propoxur. For the 0.001% w/v and 0.002% w/v concentrations, double the dose was applied to all the cockroaches in both sample. Refer to discussion for explanation.
Results: Figure 1: Log-linear graph depicting the maximal response (% of deaths) against the applied dose of propoxur (%w/v). LD50 of group data was unobtainable, while class LD50 is shown in red (0.0183% w/v).
Figure 2: Log-probit graph depicting the maximal response (% of deaths) against the applied dose of propoxur (%w/v). Group LD50 was extrapolated from the data and is shown in black (0.0076% w/v), while the class LD50 is shown in red (0.0185% w/v).
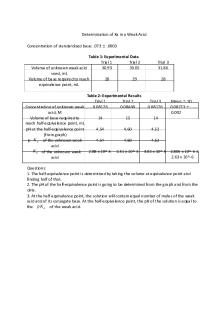
Table 1: LD50 values for group and class Group LD50
Class LD50
Log-Linear Graph
N/A
0.403 mg/kg
Log-Probit Graph
0.254 mg/kg
0.407 mg/kg
*Refer to appendix for calculations
Discussion: In the method, a major component affecting the group data was that the dose volume was doubled for the 0.001% and 0.002% concentrations. This change to the method was, rather in fact, due to human error in determining the appropriate amount of propoxur to apply to the cockroaches. Discrepancies in the method found in the Pharmacology 1 laboratory manual on pages 34-35 had led to these errors. The method required only 1µL of insecticide to be applied topically to each cockroach. However, the image on page 34 suggested that the dispenser should be rotated until a drop was visible. Since two separate 3
individuals carried out the task of applying the insecticide, this quantity was quite subjective. As such, for the 0.001% and 0.002% concentrations, the dispenser knob was rotated twice until a drop was visible, hence 2µL had been dispensed. Due to this error, the log-linear graph (figure 1) had inconsistent values. Additionally, the lowest percentage killed was 60%, and as a result, an LD50 concentration could not be obtained from the log-linear graph. The log-probit graph, seen in figure 2, produced comparably higher quality results than the log-linear graph. Particularly for the group data, where the line of best fit was extrapolated until an LD50 was obtained. Taking into consideration the errors in the method, the LD50 dose concentration was adjusted and determined as 0.254 mg/kg (refer to appendix for details). Class LD50 concentrations for figures 1 and 2 was almost identical, with the log-linear graph producing a value of 0.403 mg/kg and the log-probit generating an LD50 of 0.407 mg/kg; an insignificant difference of 0.004 mg/kg. The group data was quite inconsistent, with a decrease in deaths from the 0.002% to the 0.005% concentrations. In contrast, the class data produced a fairly positive correlation, which ultimately resulted in a similar LD50 for each graph (table 1). Since the group data possessed overall higher death percentages in comparison to the class data, a significantly stronger gradient was generated for the log-probit graph. As a result, the group had a much lower LD50 of 0.254 mg/kg; approximately 40% lower than the class LD50. Furthermore, the LD50 values of the class and group data were not greatly impacted by the weight of the cockroach, since the group average was 0.448g, while the class average weight was 0.454g. To determine the accuracy of each graph, a closer inspection and comparison between the log-linear and log-probit is vital. The log-linear graph produces a sigmoidal curve, whereas a linear relationship is illustrated by a log-probit graph. As such, log-linear graphs provide a clear pattern and relationship between the variables. However, since a curve of best fit is utilised, it is quite difficult to extrapolate the curve in order to produce additional data. In the case of the group data, the LD50 could not be obtained due to this reasoning. In contrast, the log-probit graph plots a linear relationship between the variables, which is not affected by outliers on the y-axis. This is because outliers (0% and 100%) are not plotted on log-probit graphs, and cannot skew the data, hence making it the more accurate graph. The group LD50 could be determined from this data, since the linear plot was easily extrapolated until it met at the LD50 intercept. Many aspects of this experiment were not controlled, resulting in several errors and great variability. A major source of variability came from the selection and handling of the cockroaches. Variation across the following factors: sex, weight, size, lifecycle development and overall health, would have certainly affected the lethal dose for each individual cockroach. Since the only characteristics that guided 4
selection were no wing development and an approximate length of 2cm, it is safe to assume that selection was still a major cause of data variation. Larger cockroaches, which would have most likely weighed more, would require a greater dose of propoxur to achieve cockroach death, since larger cockroaches have a higher volume of distribution. Additionally, female cockroaches are generally more tolerant/resistant to propoxur than their male counterparts (Lee et al. 1996). The crowded conditions the cockroaches were subjected to would have also most likely negatively impacted on their health. Another factor affecting health may include the lack of nutritional intake for each cockroach and possible dehydration during transport and storage. Aggressive handling of the specimen population may have also affected their health, since several cockroaches in the group sample had missing limbs, thus altering the required lethal dose. This is due to the fact that healthy and mature cockroaches are more likely to have a higher metabolic rate, relative to impaired cockroaches with missing limbs. Furthermore, other groups may have applied ice to the cockroaches for too long, resulting in weakened specimens that possibly lowered the overall LD50. It must also be noted that the genetic makeup of each individual cockroach is unique, and thus, a small degree of susceptibility or resistance may be present in certain cockroaches. Although this factor cannot be controlled, it is important to acknowledge it in the case that unexpected deaths occur, which may skew the data. For more accurate results, the aforementioned variables must be addressed to ensure that the experiment is performed in a controlled environment with controlled specimen. Each cockroach should be weighed individually in an attempt to minimise any considerable difference in weight that may alter the data. In addition to this, only one sex should be tested on, seeing as females are more resistant to propoxur (Lee et al. 1996). This selection can be ensured by the fact that males have two styli between their cerci, while females do not (Rozendaal 1997). Finally, delicate handling of the cockroaches must be ensured. This includes inspecting each cockroach for any physical injuries and minimising, or completing eliminating, the use of ice, which has the potential to kill the specimen. Any differences in mass, size or health of each cockroach should be accounted for when applying the dose, ensuring the smaller/lighter cockroaches receive the appropriate lower dosage. A larger population sample would ensure that any outliers are weeded out and do not significantly affect the final data. Published literature regarding the LD50 of propoxur against American cockroaches places it at 0.65 mg/kg (Valles et al. 1999). Despite this value being almost 60% greater than the class LD50, it must be taken into consideration that this lethal dose is according to adult male cockroaches with an average weight of 0.8g (Valles et al. 1999). The greater mass of the cockroaches used in the study accounts for the higher LD50 value. The fact that only adults, not nymphs, were used will also significantly affect the LD50, since adults may have a faster metabolic rate, and can therefore metabolise and eliminate the propoxur more efficiently. The study also ensured that only males were used, reducing the variables of the experiment. Furthermore, the study was performed on 200 cockroaches and was conducted by, at most, three 5
individuals (Valles et al. 1999). The class data was obtained from seven groups, with each group testing dose concentrations on 40 cockroaches. Of course, with so many students conducting the experiment, there would have been many uncontrolled variables. As such, the class LD50 was quite different from the published value. Although there were no deaths in this group’s control variable, two other groups had deaths in their control. Malnutritioned specimen, improper handling, and excessive use of ice to anaesthetise the cockroaches are possible factors that may account for these deaths. Since only three control cockroaches died across two groups (refer to appendix), it is possible that the dispenser was not properly washed with EMK. As a result, traces of the 0.1% dose concentration from previous groups may have been present, causing the deaths in the control samples. Another group’s sample, which did not have any deaths in the control, had a 100% death rate for both the 0.001% and 0.005% concentrations. A probable cause of this may have been that a large volume of each dose was added to the cockroaches in the samples. This could have occurred due to a misunderstanding of the method, and confusion between the appropriate amount of propoxur to apply (as was the case with this group). However, these outliers eventually averaged out since three other groups had approximately 10% and 20% deaths for both these concentrations.
Conclusion: The LD50 of propoxur against nymphal American cockroaches was 0.254 mg/kg, according to this group’s data. The class LD50 was 0.407 mg/kg, which is the more accurate value when compared with the literature value of 0.65 mg/kg. However, it was observed that an increasing dose of propoxur resulted in an increase of deaths in the cockroaches. Furthermore, different species and even different individuals within a species may exhibit increased genetic resistance or susceptibility to this carbamate insecticide. Although many variables are uncontrollable, it is important to recognise them when attempting to determine the LD50 of a drug. Further studies may be needed in order to confirm the LD50 of propoxur against American cockroaches. If this pest begins to show resistance, other insecticides may have to be examined as possible replacements.
6
References: Cannon, J.G. 2007, Pharmacology for Chemists, 2nd edn, Oxford University Press, New York. Colovic, M.B., Krstic, D.Z., Lazarevic-Pasti, T.D., Bondzic, A.M., Vasic, V.M. 2013, ‘Acetylcholinesterase Inhibitors: Pharmacology and Toxicology’, Current Neuropharmacology, vol. 11, no. 3, pp. 315–335. Fukuto, T.R. 1990, ‘Mechanism of action of organophosphorus and carbamate insecticides’, Environmental Health Perspectives, vol. 87, pp. 245–254. Jacobs, A. 2013, American Cockroaches - Department of Entomology, viewed 26 May 2017,
Kassiri, H., Kassiri, A., Kazemi, S. 2014, ‘Investigation on American cockroaches medically important bacteria in Khorramshahr hospital, Iran’, Asian Pacific Journal of Tropical Disease, vol. 4, no. 3, pp. 201–203. King, A.M., Aaron, C.K. 2015, ‘Organophosphate and Carbamate Poisoning’, Emergency Medicine Clinics of North America, vol. 33, no. 1, pp. 133–151. Lee, C.Y., Yap, H.H., Chong, N.L. 1996, ‘Insecticide toxicity on the adult German cockroach, Blattella germanica (L.) (Dictyoptera: Blattellidae)’, Malaysian Journal of Science, vol. 17, pp. 1–9. Robinson, W.H. 2005, Urban Insects and Arachnids: A Handbook of Urban Entomology, Cambridge University Press, Cambridge. Rozendaal, J.A. 1997, Vector Control: Methods for Use by Individuals and Communities, Alden Press, England. Valles, S.M., Koehler, P.G., Brenner, R.J. 1999, ‘Comparative insecticide susceptibility and detoxification enzyme activities among pestiferous Blattodea’, Comparative Biochemistry and Physiology Part C: Pharmacology, Toxicology and Endocrinology, vol. 124, no. 3, pp. 227–232.
7
Appendix: Class average weight of cockroach: 0.454g Group average weight of cockroach: 0.448g Log-linear LD50 calculations: Class: 0.0183% w/v = 0.0183g/100mL
LD50
Convert g/mL to mg/μL
= 0.0183 mg/100μL
Find the mass per 1μL
= 0.000183 mg/μL
(Since ~ 1μL of propoxur was applied to the cockroaches,
= 0.000183 mg/0.454g
simply substitute μL with the average weight of a cockroach)
= 0.000403 mg/g
Convert from g to kg by multiplying by 1000
= 0.403 mg/kg
Log-probit LD50 calculations: Class: 0.0185% w/v = 0.0185g/100mL
LD50
Convert g/mL to mg/μL
= 0.0185 mg/100μL
Find the mass per 1μL
= 0.000185 mg/μL
(Since ~ 1μL of propoxur was applied to the cockroaches,
= 0.000185 mg/0.454g
simply substitute μL with the average weight of a cockroach)
= 0.000407 mg/g
Convert from g to kg by multiplying by 1000
= 0.407 mg/kg
Group (with extrapolated dose concentration): 0.0076% w/v = 0.0076g/100mL
LD50
Convert g/mL to mg/μL
= 0.0076 mg/100μL
Find the mass per 1μL
= 0.000076 mg/μL
(Since ~ 1.5μL* of propoxur was applied to the cockroaches,
= 0.000076 mg x 1.5
multiply the mg by 1.5)
= 0.000114 mg/0.448g
Substitute μL with the average weight of a cockroach)
= 0.000254 mg/g
Convert from g to kg by multiplying by 1000
= 0.254 mg/kg
*2μL + 2μL + 1μL + 1μL = 6μL/4 = 1.5μL Double dose was applied to the following concentrations: 0.001% and 0.002%, hence average dose applied to the cockroaches is 1.5μL.
8
9...
Similar Free PDFs

Determination of Ksp CuIO3
- 5 Pages

Experimental Determination of π
- 3 Pages

Iodometric Determination of Copper
- 13 Pages
Popular Institutions
- Tinajero National High School - Annex
- Politeknik Caltex Riau
- Yokohama City University
- SGT University
- University of Al-Qadisiyah
- Divine Word College of Vigan
- Techniek College Rotterdam
- Universidade de Santiago
- Universiti Teknologi MARA Cawangan Johor Kampus Pasir Gudang
- Poltekkes Kemenkes Yogyakarta
- Baguio City National High School
- Colegio san marcos
- preparatoria uno
- Centro de Bachillerato Tecnológico Industrial y de Servicios No. 107
- Dalian Maritime University
- Quang Trung Secondary School
- Colegio Tecnológico en Informática
- Corporación Regional de Educación Superior
- Grupo CEDVA
- Dar Al Uloom University
- Centro de Estudios Preuniversitarios de la Universidad Nacional de Ingeniería
- 上智大学
- Aakash International School, Nuna Majara
- San Felipe Neri Catholic School
- Kang Chiao International School - New Taipei City
- Misamis Occidental National High School
- Institución Educativa Escuela Normal Juan Ladrilleros
- Kolehiyo ng Pantukan
- Batanes State College
- Instituto Continental
- Sekolah Menengah Kejuruan Kesehatan Kaltara (Tarakan)
- Colegio de La Inmaculada Concepcion - Cebu