PHYS 1000 - Unit 3 Notes PDF

| Title | PHYS 1000 - Unit 3 Notes |
|---|---|
| Course | Astronomy 1 |
| Institution | University of Windsor |
| Pages | 7 |
| File Size | 442.5 KB |
| File Type | |
| Total Downloads | 75 |
| Total Views | 154 |
Summary
Unit 3 Notes to help you with important information from the chapter....
Description
5.1 The Behaviour of Light Interpret the light and other types of Electromagnetic Radiation given off by these objects. What is “Electromagnetic Radiation”? Radiation: a process in which energy is transmitted from one point to another in space without need for any physical connection between those 2 points. Electromagnetic: refers to the energy (Ex: light) traveling in the form of oscillating electric and magnetic fields (wave) that is given off by electrons inside atoms. Electricity and Magnetism highly related: - A changing magnetic field can produce an electric current - Changing electric currents and produce a magnetic field - Oscillating charged particles can cause an electromagnetic wave. Not until mid – 1800s: - Scientists began to recognize all typed of EM radiation could travel through space in the form of waves. - So, light/other EM waves come from atoms within stars and other objects and travel through empty space - Studying the EM waves can tell us things about the objects - The electromagnetic wave will take time to travel to us. All EM waves travel at 3 x 10^8 m/s in a vacuum. What is a Wave?: - A disturbance in which energy is transferred from place to place without physical movement of material from one place to another - Waves are usually repetitive patterns that originate with a source (atom) that is oscillating/vibrating as it absorbs, then releases energy - The wave travels, not the medium
Frequency: number of complete waves or cycles (wavelengths) that pass a given point per second (c/s = Hz)
f = # cycles total time Period: time between the passage of successive crests/troughs – time for 1 wave to pass (T) = time needed 1 complete wave Period (T) and Frequency (f) are mathematically reciprocals of each other: f = Hz = c while T = s f=1 or T=1 s c T f Velocity of a wave is the speed and direction it travels in V=f On microscopic scales, electromagnetic radiation can behave as a particle. We call these “particles” photons. The energy of a photon is proportional to the frequency of the radiation. - Light will appear less bright as it gets further from its source - Area covered is proportional to the square of the distance 5.2 The Electromagnetic Spectrum
What is temperature? How does it affect EM wave emission? Temperature of an object: direct measure of the average amount of microscopic motion within it.
- Cooler an object, slower its particles move, the less intense the energy radiated. - Hotter an object, faster its particles, the more intense the energy radiated. No natural object Radiates all its Energy at just 1 Wavelength - Energy instead given off over a range of frequencies (wavelengths) - Intensity of energy at some wavelengths stronger than at others. - We can see that as temperature increases the peak wavelength decreases We can use to determine the temperature of an object, as in example 5.3 (p. 159) Stefan-Boltzmann law: The amount of energy radiated by an object is proportional to the fourth power of its temperature. - EX: If star A has 3 times the temperature of star B, how much more energy is it outputting? EX 5.4 in textbook 5.3 Spectroscopy in Astronomy This applies to stars as well… Hot stars are much more intense in their radiation of all energy of all energy types than cool stars. - Light can reflect off a surface - It can also be bent, or refracted when it passes from one material to another (i.e. air to glass) White light undergoes dispersion when passing through a prism due to the different wavelengths of the colours. - Dispersion: separation of different wavelengths of white light through refraction of different amounts. A spectrometer is used to disperse light for study.
When we use a spectrometer to split light, we get different spectra, depending on the light source. Summarized by Gustav Kirchoff’s Laws of Spectra: Law #1) Continuous Spectrum: - A sull continuous sequence of blended colours from red through to violet (all wavelengths in visible light range are emitted by object). - Produced by: Luminous solids/liquids or dense gases (Ex: white hot filament of a light bulb or the core of a star). Law #2) Emission Line Spectrum: - A series of specific wavelengths emitted by particular atoms - Produced by: Low-Density Hot Gas (Ex: Nebula energized/glowing from star formation). - Each element has its own unique emission spectrum. Law #3) Absorption Line Spectrum: - If a continuous spectrum passes through a cool gas, atoms of the gas will absorb the same frequencies they usually emit. - Produced by: Low-density cool gas - We see absorption lines in the spectrum of our sun
5.4 The Structure of the Atom Rutherfords experiments showed there was a positive charged nucleus (protons) and they believes negatively charged electrons orbited it.
5.5 Formation of Spectral Lines Hydrogen is the simplest example:
Heavier elements have neutral neutrons in the core as well.
There are only certain orbits that are possible for the electrons, relating to certain energy levels. Each atom has its own energy levels. If an electron is “excited” with the right amount of energy (Ex: absorbing the energy), it will jump to a higher energy level.
When the electron drops back down to a lower energy level it will emit energy in the form of radiation. The electron can cascade down.
How does this explain spectra?: - If an electron absorbs enough energy it can actually be removed from the atom. - This is known as ionization and the left over atom will now have an overall positive charge. Ionized atoms have their own (different) energy levels. 5.6 The Doppler Effect The Doppler Effect describes how relative motion between an observer and object impacts what we see. This is described by the formula: Although the spectrum will be shifted by the doppler effect, it will still be recognizable. If an atom is moving towards us when an electron changes orbits and produces a spectral line, we see that line shifted slightly toward the blue of its wavelength in a spectrum. If the atom is moving away, we see the line shifted toward the red. Key Terms: Energy Flux: the amount of energy passing through a unit area per second; the units of flux are watts per square meter. Excitation: the process of giving an atom or an ion an amount of energy greater than it has in its lowest energy (ground) state Inverse square law: the amount of energy flowing through a given area in a given time decreases in proportion to the square of the distanced from the source of energy or light Wien’s law: formula that relates the temperature of a blackbody to the wavelength at which it emits the greatest intensity of radiation
Unit 3 Test:
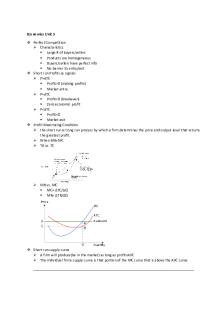
1. Consider this diagram. Which statement is true? The amplitude is 4 and the wavelength is 6. 2. In the Bohr model of the atom, an electron can only exist in specific, well-defined energy levels. True 3. The two forms of electromagnetic radiation that experience the least atmospheric opacity are: Visible light and radio waves 4. The greater the disturbance of the medium, the higher the amplitude of the wave. True 5. For hydrogen, the transition from the first to third excited state produces: A blue green absorption line. 6. The radiation our eyes are most sensitive to is the color: yellow-green at about 550nm. 7. A jar filled with gas is placed directly in front of a second jar filled with gas. Using a spectroscope to look at one jar through the other you observe bright emission spectra lines. The jar closest to you contains: the hotter gas. 8. If an object producing light is approaching us at 3,000km/sec, then all its waves are: blue shifted by 1%. 9. The longer a wave’s wavelength, the greater its energy. False 10. Which of these is the same for all forms of electromagnetic radiation in a vacuum? Speed 11. If a wave’s frequency doubles and its speed stays constant, its wavelength: is halved. 12. Which of the following statements is true of a blackbody? Its energy peaks at the wavelength determined by its temperature. 13. The Sun’s blackbody curve peaks in the Visible portion of the spectrum. 14. As white light passes through a prism, blue (shorter) wavelengths bend more than the red (longer) wavelengths, forming the rainbow of colors. True 15. When an electron in a hydrogen atom drops from the second to the first excited energy state it emits a bright red emission line called hydrogen alpha. True 16. Wien’s law states that if temperature of the blackbody is doubled, the wavelength of the peak energy will be Halved . 17. The speed of all wavelengths of light in a vacuum is: 300,000 km/sec. 18. If the electric field is changed it will have no effect on the magnetic fields of a body. False. 19. The intensity of a 120W light bulb observed from a distance 10 m away is 2.4 W/m2. What would be the intensity if this was doubled? 20. Increasing the temperature of a blackbody by a factor of 3 will increase its energy by a factor of: 21. The typical carrier frequency for FM (frequency modulation) radio’s has a wave that is vibrating one hundred times per second; this means that it has a frequency of one hundred Megahertz ....
Similar Free PDFs

PHYS 1000 - Unit 3 Notes
- 7 Pages

LAB 3 PHYS 1429
- 3 Pages

Lecture 3 Phys 2130
- 6 Pages

Comm 1000 3 - Lecture notes 16-22
- 11 Pages

PHYS Lab Report 3
- 7 Pages

PHYS 1 Assignment 3
- 6 Pages

Unit 3 Textbook Notes
- 29 Pages

Sdm-unit 3 - notes
- 35 Pages

UE18CS313 UNIT 3 Notes
- 47 Pages

HHD UNIT 3 - notes
- 43 Pages

Comm 1000 Notes
- 18 Pages

DDU-1000-Z1 - notes
- 25 Pages
Unit 3 Notes
- 7 Pages

PHYS 273 FULL Notes
- 97 Pages

Phys 91173 electrcity notes
- 8 Pages
Popular Institutions
- Tinajero National High School - Annex
- Politeknik Caltex Riau
- Yokohama City University
- SGT University
- University of Al-Qadisiyah
- Divine Word College of Vigan
- Techniek College Rotterdam
- Universidade de Santiago
- Universiti Teknologi MARA Cawangan Johor Kampus Pasir Gudang
- Poltekkes Kemenkes Yogyakarta
- Baguio City National High School
- Colegio san marcos
- preparatoria uno
- Centro de Bachillerato Tecnológico Industrial y de Servicios No. 107
- Dalian Maritime University
- Quang Trung Secondary School
- Colegio Tecnológico en Informática
- Corporación Regional de Educación Superior
- Grupo CEDVA
- Dar Al Uloom University
- Centro de Estudios Preuniversitarios de la Universidad Nacional de Ingeniería
- 上智大学
- Aakash International School, Nuna Majara
- San Felipe Neri Catholic School
- Kang Chiao International School - New Taipei City
- Misamis Occidental National High School
- Institución Educativa Escuela Normal Juan Ladrilleros
- Kolehiyo ng Pantukan
- Batanes State College
- Instituto Continental
- Sekolah Menengah Kejuruan Kesehatan Kaltara (Tarakan)
- Colegio de La Inmaculada Concepcion - Cebu
