Solar Energy Recap PDF

| Title | Solar Energy Recap |
|---|---|
| Course | Solar Energy |
| Institution | Technische Universiteit Delft |
| Pages | 40 |
| File Size | 2.7 MB |
| File Type | |
| Total Downloads | 64 |
| Total Views | 143 |
Summary
Samenvatting Solar Energy...
Description
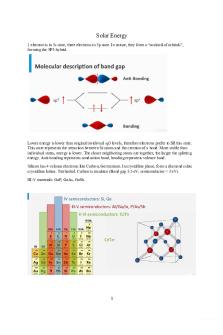
Solar Energy 1 electron is in 3s state, three electrons in 3p state. In nature, they form a “cocktail of orbitals”, forming the SP3-hybrid.
Lower energy is lower than original invidivual sp3 levels, therefore electrons prefer to fill this state. This state represents the attraction between Si atoms and the creation of a bond. More stable than individual states, energy is lower. The closer neighboring atoms are together, the larger the splitting energy. Anti-bonding represents conduction band, bonding represents valence band. Silicon has 4 valance electrons like Carbon, Germanium. In crystalline phase, form a diamond cubic crystalline lattice. Tetrhedral. Carbon is insulator (Band gap 5.5 eV, semiconductor < 3 eV). III-V materials: GaP, GaAs, GaSb.
1
Band gap related to lattice constant.
Fermi level: energy level at which the electrons have a 50% chance to occpy the energy level at any given time.
n- and p-doping: intentionally put impurity in to the silicon network (one valance electron more than used base element is n-doping). n-doping causes donor states with a forbidden energy for the band gap. The energy level is closer to the conduction band than to the valence band. This means it requires much less energy for an electron to jump from the donor state to the conduction band than from the valance band. If electrons are majority charge carriers, the Fermi level will be closer to the conduction band. 2
p-doping causes acceptor state with a forbidden energy for the band gap.
Electric field current density (left), Fick’s Law of Diffusion right. Latter can be rewritten for holes.
1. Radiative recombination a. An electron and hole recombine together, their energy is released as a photon. (Energy of at least the bandgap), 2. Auger recombination a. The energy released by recombination of an electron-hole pair is transferred to a neighboring free electron in the conduction band. This electron is excited, and loses its energy as heat to the lattice. 3. Shockley-Read-Hall (SRH) recombination a. Semiconductor materials can have various lattice defects. This defects can have forbidden states in the band gab, like doping. An electron can be trapped in such defect state. If freely moving holes find these trapped electrons, they can recombine These loss mechanisms define the life time of an electron. High recombination, low lifetime. L = srqt (D * tau)
3
In a doped material: diffusion length of minority charge carriers is less than the diffusion length of the majority charge carriers.
Depletion zone, diffusion, drift, forward bias, fixed background charges (p region (more holes) has fixed negative charges). Width of depletion zoned becomes smaller as a result of the forward bias.
4
Series resistance as small as possible, shunt as high as possible:
5
Increasing the series resistance will decrease the slope around the open-circuit voltage. The maximum power will be smaller by increasing the series resistance. Ergo: smaller fill factor. Decreasing the shunt resistance will increase the slope around the short-circuit current density. The smaller the shunt resistance, the smaller the FF.
1. Increase irradiance, aka the generation rate of charge carriers, will increase the open circuit voltage. 2. Larger the lifetime of the minority charge carrier, the larger the open-circuit voltage can be. a. Longer the life time, the larger the splitting between the quasi-Fermi levels is possible and the larger fraction of the band gap energy can be utilized. SRH recombination depends on the defect density. Simplest approximation: tau inverse proportional to defect density N_t. Defects can be located in the bulk of semiconductor materials, but can also be present in semiconductors, transparent conductive oxides and metal contacts. Let’s consider solar cells, without bulk and interface defects. That means Auger and radiative recombination are dominant. Auger recombination rate R:
Approx: tau = 1 / (charge density)^2. This means that Auger becomes dominant in materials with high densities of charge carriers (highly doped c-Si), or solar cells under strong illumination. 6
Radiative recombination:
If we ignore SRH by defects, the radiative combination for indirect band gap materials (c-Si) is inefficient and recombination will be dominated by Auger. For direct such as GaAs, under moderate illumination, radiative will be the dominant loss mechanism of charge carriers. For very high illumination, Auger starts to play a role as well. Summary: -
Defect rich cells: open-circuit voltage limited by SRH. Defect free, indirect band gap: open-circuit voltage limited by Auger Defect free, direct band gap: open-circuit voltage limited by Radiative.
We want charge carriers to be separated at the p-n junction or the back contact. But all charge carriers generated at a depth greater than the diffusion length from the p-n junction cannot be collected. Diffusion length minority charge carrier > thickness solar cell
Above: optical losses due to mismatch band gap and solar spectrum limit the maximum conversion to 48%. 7
Shockley-Queisser limit: -
Solar cell is at a certain T, it will act as black-body radiator itself and emit light in the far infrared. Around 7% of incident energy of AM1.5. We do not fully utilize the band gap energy for the open-circuit voltage.
For the small band gap (left), radiative recombination losses are dominant; for the high band gap (right), energy loss below the band gap is dominant. Shockley Queisser: optimum of 33% for band gaps in range of 1eV to 1.5eV. Note: -
Uses simplifications: recombination only due to radiation, solar cell does not increase in temperature. Limit never reached in reality: losses like reflection, parasitic absorption, electrical losses (SRH, Auger). Limit only for single-junction. Can possibly be surpassed, for example, by using multijunction.
Absorption coefficient varies per material per wavelength. For example, silicon: blue light fully absorbed within a few nanometers, red light requires path of 60 microns. Infrared after 100 microns, 10% absorbed. Losses: -
Shading Reflection at front interface. Light passing through an interface between two media with different refractive indices, will always be partly reflected. Parasitic losses, due to absorption in non-active PV layers. Transmission (starts to play for thin films). Aka cell is not thick enough.
8
P polarized light: electric field orientation oscillating parallel to plane of incident -
Has an angle at which the reflection is equal to zero = Brewster angle
S polarized light: electric field orientation oscillating perpendicular to plane of incident Reduction of refraction can be achieved by introducing an interlayer with a refractive index n_1 = sqrt( n_0 * n_2) One can design an anti-reflection coating based on destructive interferention. d = lamda / (4n_1) Total amplitude of the outgoing wave is smaller, and total irradiance coupled out of the system is smaller. Different approach: textured interfaces. Light reflected back at angles in which the trajectory of the light ray is incident somewhere else on the interface.
Monocrystalline silicon (no grain boundaries) charge carriers have longer lifetime than polycrystalline / multi-crystalline (grain boundaries, lattice mismatches) charge carriers, due to SRH. How do we make this? Lowest quality of silicon is metallurgical silicon, made of Quartzite (rock of pure silicon oxide). -
Removing oxide by furnace, melting Quartzite, mixed with carbon, split in carbon monoxide and metallurgic silicon, purity around 98%. Most used for aluminum industry. 1% for electronic grade silicon.
Next level purity: metallurgical silicon rods of polysilicon (1) Siemens a. Metallurgical silicon powder exposed to hydrogen chloride, cooled and liquified, remove impurities using distillation, vaporized and mixed with hydrogen gas, reacts with hot rods at temperature ~100 degrees Celsius, deposited on rid. b. “Chemical vapor deposition” c. Consumes a lot of energy (2) Fluidized bed reactors (FBR) 9
a. Operates at lower T b. Purity as high as one out of a million not Si atom (3) Upgraded metallurgical silicon a. Blowing gasses through Si melt to remove impurities. b. Low purity, but cheap. Polysilicon rods monocrystalline (1) Gzochralski a. Crystal in p- or n-doped silicon, molted, cylinder, relatively high oxygen (2) Float zone process a. End of rod heated, molten silicon not in contact with quartz, but with insert gas (example: Argon). (3) Silicon can be doped by adding doping gasses a. Diborane for p-doped b. Phosphine for n-doped Multi-crystalline: silicon casting. Melt highly purified silicon and pour it in a cubic shaped growthcrucible. The molten silicon solidifies into mulit-crystalline. Ingots Wafers (1) Sawing a. Waste a significant fraction as the kerf loss (thickness of wire or saw, ~100 microns, wafers ~150-200 microns). Damages, so followed by polishing. (2) Silicon Ribbon a. No losses. High temperature resistance string, pulled out of the melt. b. Not as good as good as monocrystalline silicon.
Design c-Si solar cell The cell is based on a p-type silicon wafer. The n-layer here is called the emitter layer. This emitter layer is much thinner than the wafer. (1 micron vs 100-300 microns). In the first 10 microns, most charge carriers are excited. By making the front emitter layer very thin, a large fraction of the light excited charge carriers generated by the light are created within the diffusion length of the p-n junction. Emitter layer is made by solid state diffusion: Wafers in furnace, dopant atoms present (phosphine), react with surface, will diffuse into wafer, dopants penetrate into the solid to establish desired emitter thickness. At the p-n junction, the light excited minority charge carriers are separated at p-n junction. Minority electrons in p-layer drift to n-layer. These electrons have to be collected. Since silicon n-emitter is not conductive, we need metal contacts. Metal contacts on emitter layer, cheap aluminum. The electrons have to diffuse laterally through the emitter layer to the contact to be collected. 10
Challenges: -
High lifetime guarantees large open-circuit voltage. Challenge: reduce recombination losses.
SRH: Bare c-Si surface contains many defects. The surface silicon atoms have valence electrons which cannot make a molecular orbital with the absent atoms. ▪
-
Reduce defects at surface. Thin layer of different material on top of surface, which partially restores bonding environment of silicon atoms. Must be an insulator to force the electrons to remain and move through the emitter layer. Si-oxide and Si-nitride. ▪ Reduce minority charge carrier density near surface. Increased doping of emitter layer would decrease the density of minority charge carriers, leading to lower recombination velocity. However, this is in competition with diffusion length of minority charge carriers. Challenge: metal emitter interface cannot use an insulation passivation layer, because we need electrons to conduct from the semiconductor to the metal. metal-semiconductor interface has more defects and an unwelcome high recombination velocity. Also increases contact resistance (don’t ask). ▪ High doping levels reduce recombination velocity and reduce contact resistance. Consequence: it is preferred to reduce the area of interface between metal and semiconductor to minimize recombination, and to have the emitter under the contact as heavily doped as possible. Call this ‘N++’.
▪
-
The grid pattern looks like this: a road map for electrons. ‘Highway’ is called the busbars, ‘country roads’ called fingers. Fingers have above given resistance R. Fingers will act as series resistance. Since metal contacts are at the front surface, they are unwelcome shading objects keep them as small as possible. small as possible width, high as possible height. Back surface: the holes are collected at the back contact. Electrons are the only charge carriers that exist in metal, so the holes recombine at the electrons at the contact interface. If distance between p-n interface and back contact is smaller than typical diffusion length for minority electrons, the electrons can be lost at the defects at the back contact due to SRH. Challenge: reduce SRH. ▪ Reduce contact area. Make point contacts. Rest of surface passivated by insulating passivation layer. ▪ Reduce by so-called back surface field. Higher p-doped region placed above point contacts, ‘P++’.
11
▪
P interface between p and p++ acts like a p-n junction. A barrier for the light excited minority electrons in the p to diffuse to the back-surface.Space charge field behaves like a passivation of defects at back contact interface and allows to have higher levels for the electron minority density in the p.
High-efficiency concepts. Bulk recombination low high efficiency based on mono crystalline wafer
-
-
Martin Green late 80’s: PERL aka Passivated Emitter Rear Locally diffused. 25% conversion efficiency Optical losses minimized using three concepts o Top surface is textured (pyramid). Enances total amount of light coupled into the solar cell. o Inverted pyramid structures are covered by double-layer anti-reflection coating (ARC) low top surface reflection. Often: double-layer of Mg-Fluoride and ZnSulfide. o Contact area at front side has to be as small as possible reduce shading. Fingers processed using photolithography. Emitter layer : highly doped under contacts, using heavily phosphorous regions. The rest moderately doped, to preserve an excellent “blue response”. Passivated with Si-oxide on top of emitter to suppress recombination velocity, open-circuit voltage > 700mV. Rear surface: point contacts with thermal oxide passivation layers. Oxide is passivation layer of the non-contacted area, to reduce surface recombination. Highly doped boron region as local back surface field, to limit minority electron recombination at metal contact.
SunPower: interdigitated back contact solar cell (IBC). -
Principle: does not suffer from shading losses. Advantage is that you can use monocrystalline float-zone n-type wafers. o N-type wafers do not suffer from light-induced degradation (in p-type, boron and oxygen are present, which under light pressure makes complexes that act like defects. Reduced output 2-3%). o Not sensitive for impurities like iron impurities. o less efforts processing cheaper 12
P-doped advantage: boron doping is more homogeneously distributed electric properties of n-type can vary within the wafer lowers yield Instead of one large p-n junction, multiple localized junctions. Holes separated at junction of o
-
-
-
Advantage: cross-section of metal fingers can be larger to reduce resistive losses. Passivation layer has low index such that it operates as backside mirror more absorption for >900nm Surface recombination velocity in front surface determined by minority charge carriers (holes) we have to creat a front surface field higher n-doped region “n+”. Interface between n and n+ acts like p-n. Acts like a barrier for light-excited minority holes in the lower doped region to diffuse to the front surface. 24.2%
Hetero junction: As we know, in the concept of p-n junctions the junctions are fabricated by different doping types within the same semiconductor material. band gap is the same. homojunction. Different semiconductor materials, p-doped and n-doped heterojunction.
13
Crystalline wafer based heterojunction cells -
-
-
-
-
-
N-type float zone monocrystalline silicon wafer and hydrogenated amorphous silicon. Latter: atoms are not ordered in crystalline lattice but in a disordered lattice, has higher band gap (see thin-film). Valance band is higher for p-type amorphous minority charge carriers (holes) to drift to ptype silicon. Holes experience small barrier tunneling.
Two junctions: o P-doped amorphous silicon (blue) and 5 nm of intrinsic amorphous silicon (red) o N-doped amorphous silicon (yellow) and thin layer intrinsic amorphous silicon (red) High-quality wafers, like n-type float-zone monocrystalline silicon wafer, recombination of charge carriers of surface determines the lifetime of charge carriers. Advantage: o Amorphous silicon acts as good passivation material. (Highest lifetimes accomplished). o c-Si wafer highest open-circuit voltages among crystalline >750mV o Same contact scheme at back as front o Amorphous layers deposited cheap and using low T. o Use of n-type wafers. Travel to contact? o P-doped amorphous conducts poorly diffusion lengths so small that practical metal finger spacing cannot be achieved use thin layer of ITO. 25%.
Shading, series connected, sixth cell shaded. -
Series: current limited by cell producing the lowest current. Constant load voltage over module drops Non-shaded are forced to produce high V act as reverse bias source (dotted line) Shaded cell does not generate, but dissipates energy. Warmer material cracks. Prevented by bypass diodes.
14
Thin film: III-V based absorber layers. Compared to silicon: larger (~x2), higher efficiency
Absorption coeff GaAs larger than Si to absorb same amount of life, thickness of GaAs film can be more than an order of magnitude thinner. Bandgap is relatively charge absorption coeff increases quickly above the band gap energy (?) SRH Recombination can be kept low as the processes result in high purity films. Conversion efficiency high: use different band gap energies to fully utilize band gap energy. (negate the Shockley-Queisser limit).
15
Germanium: 0.67 eV GaAs: 1.4 eV GaInP2: 1.86 eV
Connected in series (Voltages vary). Current determined by cell delivering the lowest current. Three p-n junctions in series means double p-n junctions (n-p junctions) Would lower voltage of total triple junction. Can be prevented by inclusion of ‘tunnel junction’.
-
This provides low electrical resistance and has a high band gap to prevent parasitic absorption. Relatively thin, meaning that the valence band at one side is lined up with the conduction band at the other side of the tunnel junction. Depletion zone of such junction extremely narrow Slope between valence and conduction so steep that electrons from n layer are so small that they tunnel through the small barrier to the p-layer.
Processing: High quality using epitaxy: crystalline overlayer deposited on crystalline substrate, where overlayer adopts that crystal lattice of its substrate. GaAs grown on Ge layer by layer growth without vacancy defects. High vacuum no purities.
16
Bottom cell is Ge, on top you would like junction with same lattice and higher band gap (GaAs), so they make good interfaces without coordination defects (mismatched lattices). To have a reasonable current matching we the desired band gap of the top cell should be around 1.8 eV. So, GaInP2. ~29.5%
EQE graph above. -
Block funtions Sharp band gaps, high absorption coeff is helpful. Bottom cell generates much more current than middle and top. This is ineffective use of the near infrared part, which can be improved when we move to quadruple junctions (metamorphic multi junctions need buffer layers that have a profiling lattice constant, connecting p-n junctions.
III-V Technology -
Expensive, so used for space technology Efficiency 37.8 % $/W can be improved by concentrating irradiance of a large are...
Similar Free PDFs
Solar Energy Recap
- 40 Pages

RET II Solar Thermal Energy
- 14 Pages

Solar Energy for Water Desalination
- 22 Pages

Week2 recap
- 43 Pages

Document - Chemistry recap notes
- 40 Pages

Energy
- 5 Pages

Document - Chemistry recap notes
- 35 Pages
Popular Institutions
- Tinajero National High School - Annex
- Politeknik Caltex Riau
- Yokohama City University
- SGT University
- University of Al-Qadisiyah
- Divine Word College of Vigan
- Techniek College Rotterdam
- Universidade de Santiago
- Universiti Teknologi MARA Cawangan Johor Kampus Pasir Gudang
- Poltekkes Kemenkes Yogyakarta
- Baguio City National High School
- Colegio san marcos
- preparatoria uno
- Centro de Bachillerato Tecnológico Industrial y de Servicios No. 107
- Dalian Maritime University
- Quang Trung Secondary School
- Colegio Tecnológico en Informática
- Corporación Regional de Educación Superior
- Grupo CEDVA
- Dar Al Uloom University
- Centro de Estudios Preuniversitarios de la Universidad Nacional de Ingeniería
- 上智大学
- Aakash International School, Nuna Majara
- San Felipe Neri Catholic School
- Kang Chiao International School - New Taipei City
- Misamis Occidental National High School
- Institución Educativa Escuela Normal Juan Ladrilleros
- Kolehiyo ng Pantukan
- Batanes State College
- Instituto Continental
- Sekolah Menengah Kejuruan Kesehatan Kaltara (Tarakan)
- Colegio de La Inmaculada Concepcion - Cebu








