Solubility Guidelines PDF

| Title | Solubility Guidelines |
|---|---|
| Course | General Chemistry I |
| Institution | Hofstra University |
| Pages | 1 |
| File Size | 32.7 KB |
| File Type | |
| Total Downloads | 69 |
| Total Views | 155 |
Summary
Solubility...
Description
Solubilities of Common Ionic Compounds in Water Soluble compounds contain • group 1 metal cations (Li+, Na+, K+, Rb+, and Cs+)
and ammonium ion • the halide ions
(Cl−,
Br−,
and
I−)
− − • the acetate (C 2 H 3 O 2 ), bicarbonate (HCO 3 ), nitrate (NO 3 −), and chlorate (ClO 3 −) ions
Exceptions to these solubility rules include • halides of Ag+, Hg 2 2+, and Pb2+ • sulfates of Ag+, Ba2+, Ca2+, Hg 2 2+,
Pb2+, and Sr2+
− • the sulfate (SO 4 ) ion
Insoluble compounds contain • carbonate (CO 3 2−), chromate (CrO 4 2−),
phosphate (PO 4 3−), and sulfide (S2−) ions • hydroxide ion (OH−)
Table 4.1
Exceptions to these insolubility rules include • compounds of these anions with group
1 metal cations and ammonium ion • hydroxides of group 1 metal cations
and Ba2+...
Similar Free PDFs

Solubility Guidelines
- 1 Pages

27 Solubility-S - Solubility
- 4 Pages

Solubility Rules
- 1 Pages

Predicting Solubility
- 1 Pages
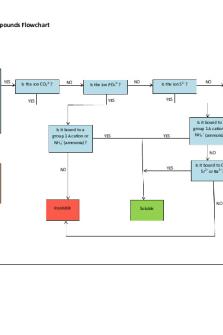
Solubility Flowchart
- 2 Pages

Solubility Rules
- 1 Pages

Solubility Rules
- 1 Pages

Solubility Table
- 1 Pages

Solubility Equilibrium
- 8 Pages

Solubility Product Lab
- 5 Pages

Solubility Worksheet Handout
- 2 Pages

Solubility rules - Exam Prep
- 1 Pages

CHM 115L RS W9 Solubility
- 6 Pages

Solubility and Solutions
- 3 Pages
Popular Institutions
- Tinajero National High School - Annex
- Politeknik Caltex Riau
- Yokohama City University
- SGT University
- University of Al-Qadisiyah
- Divine Word College of Vigan
- Techniek College Rotterdam
- Universidade de Santiago
- Universiti Teknologi MARA Cawangan Johor Kampus Pasir Gudang
- Poltekkes Kemenkes Yogyakarta
- Baguio City National High School
- Colegio san marcos
- preparatoria uno
- Centro de Bachillerato Tecnológico Industrial y de Servicios No. 107
- Dalian Maritime University
- Quang Trung Secondary School
- Colegio Tecnológico en Informática
- Corporación Regional de Educación Superior
- Grupo CEDVA
- Dar Al Uloom University
- Centro de Estudios Preuniversitarios de la Universidad Nacional de Ingeniería
- 上智大学
- Aakash International School, Nuna Majara
- San Felipe Neri Catholic School
- Kang Chiao International School - New Taipei City
- Misamis Occidental National High School
- Institución Educativa Escuela Normal Juan Ladrilleros
- Kolehiyo ng Pantukan
- Batanes State College
- Instituto Continental
- Sekolah Menengah Kejuruan Kesehatan Kaltara (Tarakan)
- Colegio de La Inmaculada Concepcion - Cebu

