Testing for non-reducing sugars PDF

| Title | Testing for non-reducing sugars |
|---|---|
| Course | Biomedical Science |
| Institution | Keele University |
| Pages | 4 |
| File Size | 134.4 KB |
| File Type | |
| Total Downloads | 73 |
| Total Views | 161 |
Summary
Inoculation of liquid and solid media following aseptic technique
SCIENTIFIC LAB REPORT...
Description
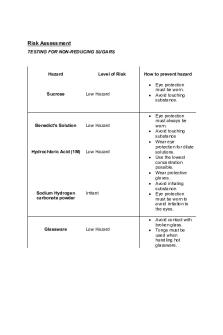
Risk Assessment TESTING FOR NON-REDUCING SUGARS
Hazard
Level of Risk
How to prevent hazard •
Sucrose
Low Hazard
•
• Benedict's Solution
Low Hazard • •
Hydrochloric Acid (1M)
Low Hazard • • •
Sodium Hydrogen carbonate powder
Irritant
•
• Glassware
Low Hazard
•
Eye protection must be worn. Avoid touching substance.
Eye protection must always be worn. Avoid touching substance Wear eye protection for dilute solutions. Use the lowest concentration possible. Wear protective gloves. Avoid inhaling substance. Eye protection must be worn to avoid irritation to the eyes. Avoid contact with broken glass. Tongs must be used when handling hot glassware.
INTRODUCTION: A non-reducing sugar is sugars that do not have an aldehyde functional group. Also due to the fact that they do not have an aldehyde group, it means that they are unable to reduce copper (I) blue to a copper (III) red. Sucrose is known to be a disaccharide that is a nonreducing sugar. The Benedict's solution is a solution to find out the presence of sugar on a sample of substances. Once the colour stays a blue colour which is the colour of the Benedict's solution, it indicates that there is no sugar present. But once the colour changes to a different colour which could be either brown, orange or brick-red, there are sugars present in the sample.
LIST OF APPARATUS: • • • • • • • • • •
5cm3 of 1% Sucrose solution Benedict's Solution 1M Hydrochloric Acid Spatula Sodium Hydrogen Carbonate powder Two boiling tubes Boiling tube tongs Two dropping pipettes Two 1cm3 Syringes Water bath for 80 Celsius
METHOD 1. 2. 3. 4.
Test the Sucrose with the Benedict's solution to ensure that it is negative. Using a clean syringe, add 1cm3 of Sucrose in a boiling tube. Add 1cm3 of the 1M Hydrochloric Acid into the tube and swirl to mix. Place the tube into the water bath to heat up for 5 minutes and remove the tube using tongs. Allow to cool.
5. Apply two spatulas of the Sodium Hydrogen Carbonate to the tube and wait until the fizzing stop.
6. When using a clean syringe, add 1cm3 of the Benedict's solution and heat it in hot water for 5 minutes. Observe any colour change.
7. Using a clean boiling tube, add 1cm 3 of Sucrose solution into it. 8. Ensure no acid is added or the sodium hydrogen carbonate powder as this is the control test tube.
9. Then heat It in the water for 5 minutes, use the tongs to remove the tubes and allow it to cool.
10. Add 1cm3 of the Benedict's solution and heat it in the water bath for 5 minutes. 11. Check for any colour change and record the results and observations.
RESULTS & DISCUSSION:
The expected results of the Benedict's test for non-reducing sugars is that the solution remaining a blue colour in the tube shows there is no non-reducing sugars and there are no sugars present. However, there were also no signs present on the other tube. Due to the case of adding the solution, the powder and Hydrochloric Acid, there were still no signs of sugars present. This might have been the case of the Sucrose concentration which may have been too low or the sucrose activity did not properly break down. Overall, the main boiling tube gave off a blue colour with a cloudy white precipitate and the control test tube had no non-reducing sugars present so the colour stayed blue.
CONCLUSION Disaccharides are hydrolysed to their constituent monosaccharides once it is boiled in diluted Hydrochloric Acid. The monosaccharide products of the Hydrolysis are reducing sugars. In conclusion, the Benedict's reagent test is for one particular reducing sugar known as Glucose. This reagent begins off with an aqua-blue colour but the presence of the glucose gives off the colour of a brick-red colour.
EVALUATION However, my results gave off a cloudy blue colour after placing it into the water bath and was should really give off a brick-red precipitate due to the glucose present in the solution. to evaluate on this, the sucrose may have been too dilute. Furthermore, we were able to give off precise results in the experiment as we followed the protocol effectively....
Similar Free PDFs
Testing for non-reducing sugars
- 4 Pages

Hypothesis Testing for dummies
- 1 Pages

PMH - testing for ketones
- 2 Pages

Testing for Language Teachers (1)
- 180 Pages

Alpha Testing Vs Beta Testing
- 11 Pages

Testing-debugging
- 24 Pages

Rockwell Testing
- 21 Pages
Popular Institutions
- Tinajero National High School - Annex
- Politeknik Caltex Riau
- Yokohama City University
- SGT University
- University of Al-Qadisiyah
- Divine Word College of Vigan
- Techniek College Rotterdam
- Universidade de Santiago
- Universiti Teknologi MARA Cawangan Johor Kampus Pasir Gudang
- Poltekkes Kemenkes Yogyakarta
- Baguio City National High School
- Colegio san marcos
- preparatoria uno
- Centro de Bachillerato Tecnológico Industrial y de Servicios No. 107
- Dalian Maritime University
- Quang Trung Secondary School
- Colegio Tecnológico en Informática
- Corporación Regional de Educación Superior
- Grupo CEDVA
- Dar Al Uloom University
- Centro de Estudios Preuniversitarios de la Universidad Nacional de Ingeniería
- 上智大学
- Aakash International School, Nuna Majara
- San Felipe Neri Catholic School
- Kang Chiao International School - New Taipei City
- Misamis Occidental National High School
- Institución Educativa Escuela Normal Juan Ladrilleros
- Kolehiyo ng Pantukan
- Batanes State College
- Instituto Continental
- Sekolah Menengah Kejuruan Kesehatan Kaltara (Tarakan)
- Colegio de La Inmaculada Concepcion - Cebu








