Virtual Atomic Spectra Lab PDF

| Title | Virtual Atomic Spectra Lab |
|---|---|
| Author | Thomas Moore |
| Course | General College Chemistry |
| Institution | Brigham Young University |
| Pages | 3 |
| File Size | 91.4 KB |
| File Type | |
| Total Downloads | 82 |
| Total Views | 164 |
Summary
Download Virtual Atomic Spectra Lab PDF
Description
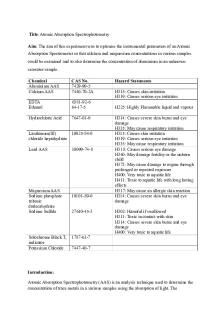
Virtual Atomic Spectra NAME ____________________________ INTRODUCTION Atoms have multiple discrete electron energy levels.
When electrons in gas phase atoms are energized, either by an electric field or by heating, they can be excited to a higher energy level within the atom. When the source of the excitation is no longer present, the electrons “relax” back down to lower energy levels by releasing energy in the form of a photon of light. Consequently, photons of different colors of light can be emitted from the same element, producing an atomic spectrum. The figure to the right shows the atomic spectrum produced by hydrogen atoms. The electron can be excited from the ground state (n=1) to any of these excited states. The energy difference between the energy levels produces a unique color of light. For example, the 656 nm light (red) is emitted from a hydrogen atom when an electron relaxes from the second excited state (n=3) to the first excited state (n=2). PROCEDURE • Open Virtual Chem Lab and go to the Quantum module. Once in the lab, go to the stockroom. Double click on the gas holder first, then get some H2 from the gas canisters. You can’t pick the gas until you take out the gas holder. Double click on the spectrometer (in the detectors section) and the electric field (in the modifiers section). • Go back to the lab and set up by placing the spectrometer in the right center location of the lab bench. Place the gas sample in the middle of the table and the electric field directly on the sample. Turn the electric field up to 300 by clicking on the tiny arrows on the upper part of the electric field. Turn on the spectrometer by clicking on the green button. Set the switches to “frequency” and “visible.” • Record your observations. Repeat these steps for Neon (Ne) and Mercury (Hg). If you leave the spectrometer and the electric field behind, you can switch out gases without having to reset the whole lab. •
•
Close the Quantum module and open the Inorganic module. Double click on a test tube to get one. Click on one of the metal ions given in the table below (sodium, barium, strontium, and copper). Then, click on the Bunsen burner at the bottom left to do a flame test. Choose the Bunsen burner without the blue cobalt screen. Click the red container to the right to clear the lab between metal ions. Watch what happens. Wait a few seconds for the color of the flame to change. Record what the final color of the flame is. Repeat for the other ions.
Colors of lines in spectrum
Purple
Number of lines observed using spectrophotometer (doesn’t need to be exact, can be too many to count) 3
Ne
Red
About 63
Hg
Blue
About 39
Red, orange, yelow, light green, green, teal, blue Red, orange, yellow, green, blue, purple
Gas in the tube
Color seen with eye
H2
Red, teal, blue
Substance in flame Strontium
Flame color Pink
Substance in flame Barium
Sodium
Yellow
Copper
Flame color White to pink to yellow Green
QUESTIONS • A fluorescent light bulb contains hot gaseous mercury atoms. An incandescent bulb contains a thin piece of metal called a filament. It is heated until it glows, a phenomenon known as blackbody radiation. Based on what you know about atomic spectra, will the fluorescent bulb or the incandescent bulb have a continuous spectrum? Explain. The flourescent lights would be continous as we already proved earlier mercury registers as continous. If you need help deciding, you can go into the virtual module. Go into the stockroom, click on the clipboard, and select the “Blackbody radiation” experiment. Flip the switch to “visible” and compare what you see to what you observed earlier with the hot gaseous elements.
•
Imagine that you are designing custom fireworks. Which metal salts (not gases) would you use to create the following colors of fireworks?
Firework
•
Color
Metal Salt(s)
Orange Red Purple
Bismuth(Bi) and Iron(Fe) Cadmium(Cd) and Mercury (Hg) Chromium(Cr) and Ammonium(NH3 )
Each element has its own distinct emission color bands. Why are the emission spectra of no two elements the same?
Each element has a different atomic spectrum, since each element radiates a certain amount of energy it results in no two elements having the same emission spectra. •
The speed of light is 2.99792 x 108 m/s. All wavelengths of light travel at the same speed. The wavelength associated with one of the colors of light emitted by hydrogen is 486.133 nm. Convert this wavelength to frequency using this relationship: c= νλ, where λ is wavelength, ν is frequency, and c is the speed of light. v= 616687 Hz
•
The energy associated with a photon of light energy (in units of Joules, J) can be calculated using the relationship E=hν where E is energy, h is Planck’s constant (6.62607 x 10-34 J·s) and ν is the frequency of the light energy. This is also the energy released as an electron relaxes from a higher energy state to a lower energy state. Calculate the energy associated with a photon that has a wavelength of 486.133 nm. E= 4.08621123x10-28-J
Optional Exploration Activity: You have learned about many important experiments in your class. In the virtual lab, you can see what these scientists saw. Check out the Millikan oil drop experiment, the Rutherford backscattering experiment, and the Thompson experiment. You can also explore the photoelectric effect and blackbody radiation. You can find these by going into the stockroom and opening the clipboard. If you need more direction, use the worksheets available in the Beyond Labz Client. This could be helpful for remembering which scientist did which experiment....
Similar Free PDFs

Virtual Atomic Spectra Lab
- 3 Pages

Atomic Spectra - Lab Report
- 9 Pages

Lab Atomic Emission Spectra
- 11 Pages

Atomic Emission Spectra Lab
- 7 Pages

CHEM 105 Atomic Spectra Lab
- 3 Pages

Pre-Lab Atomic Emission Spectra
- 2 Pages

Ex 5 Atomic Spectra - Lab report 5
- 10 Pages

Atomic Emission Spectra
- 4 Pages

LAB - Atomic Mass Candium
- 2 Pages

Atomic Emissoin Specta Pre Lab
- 2 Pages
Popular Institutions
- Tinajero National High School - Annex
- Politeknik Caltex Riau
- Yokohama City University
- SGT University
- University of Al-Qadisiyah
- Divine Word College of Vigan
- Techniek College Rotterdam
- Universidade de Santiago
- Universiti Teknologi MARA Cawangan Johor Kampus Pasir Gudang
- Poltekkes Kemenkes Yogyakarta
- Baguio City National High School
- Colegio san marcos
- preparatoria uno
- Centro de Bachillerato Tecnológico Industrial y de Servicios No. 107
- Dalian Maritime University
- Quang Trung Secondary School
- Colegio Tecnológico en Informática
- Corporación Regional de Educación Superior
- Grupo CEDVA
- Dar Al Uloom University
- Centro de Estudios Preuniversitarios de la Universidad Nacional de Ingeniería
- 上智大学
- Aakash International School, Nuna Majara
- San Felipe Neri Catholic School
- Kang Chiao International School - New Taipei City
- Misamis Occidental National High School
- Institución Educativa Escuela Normal Juan Ladrilleros
- Kolehiyo ng Pantukan
- Batanes State College
- Instituto Continental
- Sekolah Menengah Kejuruan Kesehatan Kaltara (Tarakan)
- Colegio de La Inmaculada Concepcion - Cebu