Atomic Absorption Spectroscopy lab report PDF

| Title | Atomic Absorption Spectroscopy lab report |
|---|---|
| Course | Analysis of Environmental Samples |
| Institution | Dublin City University |
| Pages | 12 |
| File Size | 383.1 KB |
| File Type | |
| Total Downloads | 98 |
| Total Views | 208 |
Summary
Detailed lab report for Practical on Atomic Absorption Spectroscopy...
Description
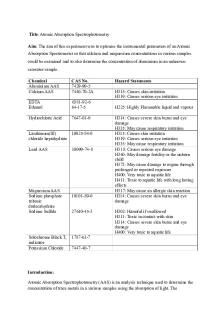
Title: Atomic Absorption Spectrophotometry Aim: The aim of this experiment was to optimise the instrumental parameters of an Atomic Absorption Spectrometer so that calcium and magnesium concentrations in various samples could be examined and to also determine the concentration of aluminium in an unknown seawater sample. Chemical Aluminium AAS Calcium AAS
CAS No. 7429-90-5 7440-70-2A
EDTA Ethanol
6381-92-6 64-17-5
Hydrochloric Acid
7647-01-0
Lanthanum(III) 10025-84-0 chloride heptahydrate Lead AAS
Magnesium AAS Sodium phosphate tribasic dodecahydrate Sodium Sulfide
Solochrome Black T, indicator Potassium Chloride
10099-74-8
10101-89-0
27610-45-3
Hazard Statements H315: Causes skin irritation H319: Causes serious eye irritation H225: Highly Flammable liquid and vapour H314: Causes severe skin burns and eye damage H335: May cause respiratory irritation H315: Causes skin irritation H319: Causes serious eye irritation H335: May cause respiratory irritation H318: Causes serious eye damage H360: May damage fertility or the unborn child H373: May cause damage to organs through prolonged or repeated exposure H400: Very toxic to aquatic life H411: Toxic to aquatic life with long lasting effects H317: May cause an allergic skin reaction H314: Causes severe skin burns and eye damage H302: Harmful if swallowed H311: Toxic in contact with skin H314: Causes severe skin burns and eye damage H400: Very toxic to aquatic life
1787-61-7 7447-40-7
Introduction: Atomic Absorption Spectrophotometry (AAS) is an analysis technique used to determine the concentration of trace metals in a various samples using the absorption of light. The
technique is used worldwide today and an important analysis tool in many industries. AAS is used throughout the medical sector for blood and soft tissue analysis and also in the research and production of pharmaceuticals. AAS also plays a huge role in the trace analysis of metals in water analysis. AAS works on the basis of atomization. When a sample is introduced to the instrument, it enters in an aerosol form. This aerosol is generated from the sample using the nebuliser. The aerosol sample then enters a possible of either two flames, an air- acetylene flame or a nitrous oxide- acetylene flame. Most AAS flames are air-acetylene flames which have a maximum temperature of 2300 K. Nitrous oxide is also used as the oxidant and this can produce a much higher flame temperature of up to 2900 K. This oxidant was used during the aluminium analysis due to the ability to reach a higher temperature and thus increase sensitivity and ionise more metal ions. As the sample enters the flame it is evaporated and atomised. This involves the flame dissociating the metal ions present in the sample to free atoms which have energy levels in the ground state (2). Due to a large percentage of atoms being in the ground state, they are available to be excited or absorbed. This excitation occurs due to the energy emitted from the hallow cathode lamp. The lamp emits a beam of radiant energy through the flame causing the ground state atoms’ energy to rise to a higher level. The lamp emits the exact wavelength required for the analysis. This is because the atoms of the metal being tested are present in the lamp. This means that when the lamp is on, these atoms are supplied with energy, causing them to elevate to the excited levels. When returning to the ground state, the exact same wavelengths are then emitted, since it is the analysed metal undergoing the excitation and the energy levels are the same(1). The light is directed at then at the flame containing the sample. The light intensity which reaches the detector through the flame is picked up and the amount of energy that is absorbed by the free state atoms from the beam is a function of the concentration of the concentration of the element within the flame. (2) The below image shows the principle components of an AAS (6)
The analytes being tested for in this experiment included Calcium, magnesium and aluminium. The presence of both Ca2+ and Mg2+ ions in the water is what leads to water hardness. Calcium and magnesium naturally occur in water and are especially prevalent in areas with limestone as the parent bedrock. Calcium also functions as a pH stabilizer due to its buffering qualities and also gives water a better taste. Calcium reacts with water at room temperature and the reaction mechanism is shown below (3). Ca (s) + 2H2O (g) → Ca(OH)2 (aq) + H2 (g) This reaction forms calcium hydroxide that dissolves in water as a soda, and hydrogen gas. When calcium and magnesium ions are heated, the bicarbonates can decompose to an insoluble form of carbonate that can build up in pipes and kettles, more commonly known as lime scale. The other method for testing for both ions uses Na2H2EDTA as a solution that is added to the sample via titration. Solochrome black indicator reacts red with magnesium ions. As the EDTA is added to the sample it first reacts with the calcium ions and then followed by the free magnesium ions in the sample. When all ions have reacted together the indicator turns blue marking the end of the reaction. EDTA is used for this experiment because it is a strong chelating agent that sequesters a variety of polyvalent cations such as calcium and magnesium (4). It can be used in the pharmaceutical industry, as a food additive and also for the removal of lime scale. Method & Instruments Used There were no deviations from the method outlined on pages 30-35 of the CS351A manual. The instrument used was a Varian SpectrAA
Quality control: For this part 3ppm calcium was used along with the calcium lamp. The acetylene pressure was set to 1 and burner height to five. Quality Control Run 1 2 3 Mean RSD Standard Deviation
Absorbance Set 1 (Abs) 0.2024 0.2022 0.2033 0.2027 0.3 0.006
Absorbance Set 2 (Abs) 0.1545 0.1554 0.1547 0.1540 0.3 0.0005
A second QC run was completed due to the first set of data resulting in a target value well above the two standard deviations on the Shewart Chart. After some adjustments to Acetylene flow and burner height the second set of absorbances resulted in a target value just below and within one standard deviation on the Shewart chart.
Results and Discussion: Part A: Investigation of Instrumental Parameters Absorbance Readings at Varying Acetylene Pressures Acetylene Pressure (psi) 0.5 1.0 1.5 2.0
Absorbance 1
Absorbance 2
Absorbance 3
Mean ± S.D.
0.0977 0.1084 0.0854 0.0594
0.0963 0.1088 0.0814 0.0583
0.0950 0.1086 0.0812 0.0586
0.0963 ± 0.0013 0.1080 ± 0.0002 0.0827 ± 0.0024 0.0588 ± 0.0005
Using a standard calcium solution, by varying the fuel-oxidant ratio, an optimum acetylene pressure was discovered. A ratio of 1.0psi resulted in the greatest absorbance of the calcium standard. As all other variables were kept constant, this meant 1.0psi was the most suited pressure and was the pressure that used for the rest of the experiment. Absorbance Readings at Varying Burner Heights Burner Height 5 10 15
Absorbance 1 0.1182 0.1063 0.0566
Absorbance 2 0.1158 0.1046 0.0572
Absorbance 3 0.1155 0.1061 0.0573
Mean ± S.D. 0.1165 ± 0.0015 0.1057 ± 0.0010 0.0570 ± 0.0004
Again a standard calcium solution was used to find the optimum burner height. A burner height of 5 resulted in the greatest absorbance of the calcium standard followed by 10 with 15 having the lowest absorbance. Consistent absorbance results with a low standard deviation meant that the results were reliable. This allowed a height of 5 to be chosen for the unknown analysis. Absorbance Readings for Interference Effects
2mg/L Ca 2mg/L Ca + 20mg/L Al3+ 2mg/L Ca + 20mg/L PO432mg/L Ca + 200mg/L PO43+600mg/L La3+ 2mg/L Ca in 50% ethanol
Absorbance 1 0.1084 0.0053
Absorbance 2 0.1088 0.0054
Absorbance 3 0.1086 0.0050
Mean ± S.D. 0.1080 ± 0.0002 0.0052 ± 0.0002
0.0967
0.0968
0.0969
0.0968 ± 0.0001
0.1362
0.1366
0.1379
0.1369 ± 0.0009
0.0700
0.0693
0.0707
0.0700 ± 0.0007
The above readings showed that the highest level of interference was caused by the aluminium ions present in the solutions. The absorbance levels were much lower than all
other mixed solutions such as phosphate and lanthanum. The aluminium ions severely affect the calcium ions from being detected. Part B: Determination of Ca and Mg in Samples by AAS Calcium Calcium Absorbance Readings at Varying Concentrations Calcium Conc (mg/L) 0.5 0.75 1.0 2.0 3.0
Absorbance 1
Absorbance 2
Absorbance 3
Mean±S.D.
0.0521 0.0781 0.1011 0.1679 0.2119
0.0520 0.0786 0.1013 0.1685 0.2149
0.0515 0.0779 0.1019 0.1690 0.2097
0.0519±0.0003 0.0782±0.0003 0.1014±0.0004 0.1684±0.0005 0.2119±0.0022
Calcium Concentration (mg/L) Vs Absorbance (Abs) 0.25 f(x) = 0.06 x + 0.03 R² = 0.98
0.2 0.15
Mean±S.D. Linear (Mean±S.D.)
0.1 0.05 0 0
0.5
1
1.5
2
2.5
3
3.5
Unknown Sample Absorbance Readings at with Required Dilutions Unknowns Tap Water (1:10 dil) Mineral Water (1:20 dil) Bisodal Tablet (1:10 dil)
Absorbance 1 0.1184
Absorbance 2 0.1188
Absorbance 3 0.1211
Mean±S.D. 0.1194±0.0015
0.1759
0.1779
0.1781
0.1773±0.0012
0.1831
0.1839
0.1816
0.1844±0.0018
The unknown concentrations all required dilutions so they fit onto the standard curve. The standard curve was then used to determine the concentration of calcium in the unknowns. Tap water => y = 0.0631x + 0.0309
0.1194 = 0.0631x + 0.0309 X = 1.402 x 10 (dilution factor) Concentration of Calcium = 14.012mg/L Mineral Water =>
y = 0.0631x + 0.0309
0.1773 = 0.0631x + 0.0309 X = 2.320 x 20 (dilution factor) Concentration of Calcium = 46.402mg/L Bisodol Tablet =>
y = 0.0631x + 0.0309
0.1844 = 0.0631x + 0.0309 X = 2.432 x 10 (dilution factor) Concentration of Calcium = 24.326mg/L from 100.4mg sample 24% Calcium content From the results the calcium concentrations varied with tap water containing the lowest concentration of calcium and mineral water being the highest concentrated. Further study of calcium levels in water showed that rivers generally contain 1-2 ppm calcium, but in lime areas rivers may contains calcium concentrations as high as 100 ppm. Drinking water’s level’s varies from 1 to 135 mg/L with most spring waters were found to have a relatively low calcium concentration, with an average of 21.8 mg/L. Mineral waters were generally found to contain higher calcium concentrations, an average of 208 mg/L of calcium. These figures are similar to the results found for this experiment with the mineral water having a below average concentration of calcium. Bisodol tablets supposedly contain 522mg of calcium carbonate per tablet from a total weight of 654mg. This shows that the calcium content is lower than what is advertised (79%). Magnesium Magnesium Absorbance Readings at Varying Concentrations Magnesium Conc (mg/L) 0.1 0.2 0.3 0.4 0.5
Absorbance 1
Absorbance 2
Absorbance 3
Mean±S.D.
0.0419 0.0778 0.1140 0.1468 0.1646
0.0416 0.0777 0.1175 0.1480 0.1657
0.0419 0.0774 0.1130 0.1483 0.1653
0.0418±0.0002 0.0776±0.0002 0.1148±0.0023 0.1477±0.0008 0.1652±0.0006
Magnesium Concentration (mg/L) Vs Absorbtion (Abs) 0.18 0.16
f(x) = 0.32 x + 0.01 R² = 0.99
0.14 0.12
ABS Linear (ABS)
0.1 0.08 0.06 0.04 0.02 0 0.05 0.1 0.15 0.2 0.25 0.3 0.35 0.4 0.45 0.5 0.55
Unknown Sample Absorbance Readings at with Required Dilutions Unkowns Tap Water (1:10 dil) Mineral Water (1:100 dil) Bisodal Tablet (1:50 dil)
Absorbance 1 0.0488
Absorbance 2 0.0489
Absorbance 3 0.0485
Mean±S.D. 0.0487±0.0002
0.1482
0.1468
0.1475
0.1475±0.0007
0.1190
0.1195
0.1195
0.1193±0.0003
Tap water => y = 0.3169x + 0.0144 0.0487 = 0.3169x + 0.0144 X = 0.1082 x 10 (dilution factor) Concentration of Magnesium = 10.82 mg/L Mineral Water =>
y = 0.3169x + 0.0144
0.1475 = 0.3169x + 0.0144 X = 0.4200 x 100 (dilution factor) Concentration of Magnesium = 42.00 mg/L Bisodol Tablet =>
y = 0.3169x + 0.0144
0.1193 = 0.3169x + 0.0144 X = 0.3310 x 50 (dilution factor)
Concentration of Magnesium = 16.55 mg/L from 100.4mg sample 16.5% magnesium content
Again from the results, the mineral water sample contained the highest concentration of magnesium with concentrations nearly four times as much as both other samples. The bisodol tablet also had a high concentration seeing as though it is advertised that the tablet contains 64mg magnesium content from a total 654mg tablet (9.78%). This shows that the tablet showed higher concentrations than advertised. Magnesium levels in drinking water in the US varied from 0.3 to 41ppm. This shows that the mineral water sample had a relatively high concentration and the tap water had a relatively normal level of magnesium in comparison. (7) Titration Volume required to neutralise EDTA Titre 1 2 3 4 5
Vol of EDTA to Neutralise (mls) 21.5 23.2 22.9 23.2 22.9
The average titre needed to neutralise the EDTA was 22.74mls V1 x M1 x n2 = V2 x M2 x n1 40.0 x M1 x 1 = 22.74 x 0.01 x 1 M1 = 22.74 x 0.01 x 1 / (40.0 x 1) M1= 0.00568 moles/litre of Ca2+ and Mg2+ (0.00568) *100 = 0.5685 g/L CaCO3 0.5685 x 1000 = 568.5 ppm CaCO3 = water hardness
This result is well above normal levels of hardness and experimental error must have played a part in these results. Hard water usually has a ppm of 180 or over (9); these results well exceed this figure. Aluminium Seawater Absorbance Readings using standard addition method
Solution Number 1 2 3 4 5
Vol Vol Al Unknow Standar n S.Water d (mL) 5 0 5 2.5 5 5 5 10 5 20
Final Vol (mL) 50 50 50 50 50
Abs 1
Abs 2
Abs 3
Mean±S.D.
0.0277 0.0410 0.0566 0.0791 0.1334
0.0274 0.0411 0.0574 0.0802 0.1280
0.0272 0.0406 0.0550 0.0781 0.1307
0.0274±0.0002 0.0409±0.0003 0.0563±0.0012 0.0792±0.0011 0.1307±0.0027
Aluminium Concentration(mg/L) vs Absorption (Abs) 0.14 f(x) = 0.01 x + 0.03 R² = 1
0.12 0.1
Absorbtion Linear (Absorbtion)
0.08 0.06 0.04 0.02 0 0
5
10
15
20
25
Seawater and Potassium Chloride Absorbance Readings using standard addition method Solution Number 1 2 3 4 5
Vol Vol Al Unknow Standar n S.Water d (mL) 5 0 5 2.5 5 5 5 10 5 20
Final Vol (mL) 50 50 50 50 50
Abs 1
Abs 2
Abs 3
Mean±S.D.
0.0389 0.0414 0.0492 0.0737 0.1129
0.0370 0.0417 0.0500 0.0736 0.1116
0.0365 0.0399 0.0487 0.0725 0.1111
0.0375±0.0012 0.0410±0.0009 0.0493±0.0006 0.0733±0.0007 0.1119±0.0009
Aluminium 0.12 f(x) = 0 x + 0.03 R² = 0.99
0.1 0.08
Mean Linear (Mean)
0.06 0.04 0.02 0 0
5
10
15
20
25
A lack of understanding of the standard addition method for determining concentrations led to no final result for aluminium concentration in seawater. However usual concentrations of aluminium in seawater can vary from between 0.013 and 5 ppb. The Atlantic Ocean is known to contain more aluminium than the Pacific Ocean. River water usually contains around 400 ppb aluminium (8).
Conclusion: To conclude, the concentrations in both the tap water and mineral water represented figures that correlated with researched average figures. The mineral water contained the highest levels of both calcium and magnesium ions. Also it was concluded is that the bisodol tablets calcium concentration is slightly below advertised levels whereas the magnesium level is slightly above. In comparison between the titration results and the AAS results for calcium and magnesium concentrations, the results from the titration seemed unrealistic and should probably be dismissed. The results from the AAS on the other hand seemed within the average data researched and could be classed as accurate. The aluminium data received showed that the addition of potassium chloride caused an interference with greater absorbance at lower concentrations of aluminium and lower absorbance at higher concentrations of aluminium. Personal Reflection: Overall in my opinion this experiment worked extremely well. Time management was improved from previous labs and we worked efficiently through the dilutions and readings with good delegation of work. If I was to repeat this experiment I would have had increased the samples of tap water and mineral water with a known source. It would have been interesting to correlate both the calcium and magnesium levels to the area and possibly
research the area/ geology/ local bedrocks. Similarly for the aluminium seawater samples, a variety of samples from different areas along a coastline could produce interesting findings. I also would have researched the standard addition method more so that my data would yield a more accurate result and I would have a greater understanding of how the calculations were to be carried out for this section.
Questions: 1) The two instrumental parameters varied for this experiment were fuel-oxidant ratio and burner height. The optimum acetylene pressure for the absorbance of Calcium was 1 psi. Above or below this figure resulted in lower sensitivity and lower absorbance. The optimum burner height was 10 with both 5 and 15 resulting in lower levels of absorbance also. As all other parameters were kept constant showing that varying these parameters reduced absorbance. 2) Free atom concentration increases with flame height (vertical position) which results in generally higher absorbance values. The greater the optical path length, the greater the absorbance. This means that the absorbance values would increase and the calibration curve would remain linear. Lamp current must be accurately chosen with too high of a value running the risk of self-absorption leading to inaccurate for absorbance readings. Increased slit width allows a higher number of wavelengths to pass through the monochromator. If a larger width is used the absorbance values would not have a relationship with concentration and so the calibration curve will not have a desired linear shape. Each element has its own unique wavelength, if the wrong wavelength is used then maximum excitation of elements will not occur meaning that radiation will not be emitted as strongly or at all, this will result in small absorbance values. Chemical modification is often used to make a sample more suitable for AAS analysis. This will improve the absorbance values obtained and lead to more improved linear calibration curves Suppression results in a lower ionization of the sample therefore decreasing absorbance values and leading to an incorrect less linear calibration curve. 3) UV-Vis Spectrophotometry
Similar to the method used for this experiment UV-VIS spectrophotometry could al...
Similar Free PDFs

Atomic Absorption Spectroscopy
- 271 Pages

Atomic Absorption Spectroscopy
- 9 Pages

Atomic Absorption Spectroscopy
- 1 Pages

LAB REPORT GAS ABSORPTION
- 11 Pages

Chem 1411 Atomic Spectroscopy
- 3 Pages

Atomic Spectra - Lab Report
- 9 Pages

Spectroscopy Lab
- 2 Pages

Ex 5 Atomic Spectra - Lab report 5
- 10 Pages
Popular Institutions
- Tinajero National High School - Annex
- Politeknik Caltex Riau
- Yokohama City University
- SGT University
- University of Al-Qadisiyah
- Divine Word College of Vigan
- Techniek College Rotterdam
- Universidade de Santiago
- Universiti Teknologi MARA Cawangan Johor Kampus Pasir Gudang
- Poltekkes Kemenkes Yogyakarta
- Baguio City National High School
- Colegio san marcos
- preparatoria uno
- Centro de Bachillerato Tecnológico Industrial y de Servicios No. 107
- Dalian Maritime University
- Quang Trung Secondary School
- Colegio Tecnológico en Informática
- Corporación Regional de Educación Superior
- Grupo CEDVA
- Dar Al Uloom University
- Centro de Estudios Preuniversitarios de la Universidad Nacional de Ingeniería
- 上智大学
- Aakash International School, Nuna Majara
- San Felipe Neri Catholic School
- Kang Chiao International School - New Taipei City
- Misamis Occidental National High School
- Institución Educativa Escuela Normal Juan Ladrilleros
- Kolehiyo ng Pantukan
- Batanes State College
- Instituto Continental
- Sekolah Menengah Kejuruan Kesehatan Kaltara (Tarakan)
- Colegio de La Inmaculada Concepcion - Cebu