Lecture notes – The procedure of atomic absorption spectroscopy PDF

| Title | Lecture notes – The procedure of atomic absorption spectroscopy |
|---|---|
| Course | Biology |
| Institution | University of Salford |
| Pages | 2 |
| File Size | 35.4 KB |
| File Type | |
| Total Downloads | 98 |
| Total Views | 121 |
Summary
Lecture notes based on The procedure of atomic absorption spectroscopy are useful for exams...
Description
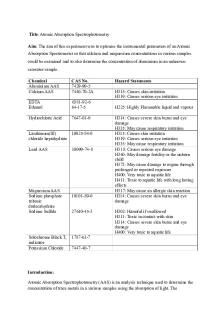
Lecture notes – The procedure of atomic absorption spectroscopy Atomic absorption is an analytical technique utilizing the principle of spectroscopy for the quantitative determination of chemical elements. The Principle of Atomic absorption spectroscopy Atomic absorption spectroscopy utilizes the principle that free electrons generated in an atomizer absorb radiation of different wavelengths. The free electrons absorb UV or visible light, causing the electrons to transfer to higher energy orbits. During this process, the absorption spectrum is released, which is detected by the photodetectors. The absorption spectrum formed allows the quantification of free electrons in the gaseous state of the matter. The amount of photon (radiation) absorbed results in an absorption spectrum which can then be measured in terms of absorbance. The absorbance of a sample is dependent on the concentration of molecules in the sample. The Steps of Atomic absorption spectroscopy The liquid sample is mixed with a particular volume of spirit which is added to a flask which is then vaporized into a gas by a fuel-rich acetylene-nitrous oxide flame. A lamp is set with the necessary wavelength as a light source. The gas formed from the liquid sample is then passed through a detector that detects the absorbance of the atoms in the gas. A similar process is performed for the detection of absorbance of solvent bank and standard solution. A graph is plotted for the absorbance against the concentration of the molecules in the sample. Uses of Atomic absorption spectroscopy Atomic absorption spectroscopy can be used for the quantitative and qualitative determination of metallic elements in biological systems. This also helps in the detection of metals as an impurity in alloys and other mixtures. Atomic absorption spectroscopy has been utilized for the purification of environmental samples like water and soil. Detection of metals in pharmaceutical products and oil products can also be done by this method. The Circular dichroism spectroscopy Circular dichroism spectroscopy is a type of light absorbance spectroscopy that measures the differences in the absorbance of right and left polarized light.
The Principle of Circular dichroism spectroscopy
Left- and right-handed polarized components of the incident light are absorbed differently by the sample, which yields a difference in the absorption coefficients. This difference is termed circular dichroism. Circularly-polarized light rays will travel through an optically active medium with different velocities due to the different indices of refraction for right- and left-circularly polarized light. Optically active chiral molecules will preferentially absorb one direction of the circularly polarized light. The peptide bonds in protein act as chromophores. The peptide bonds are the optically active chiral molecules of protein, and the number of chromophores is proportional to the magnitude of absorption. Thus, the magnitude of absorption is then used for the verification of the adopted secondary structure of proteins. Steps of Circular dichroism spectroscopy The sample is placed in a transport vessel with buffers which is then placed in the spectrometer. In the spectrometer, left and right circularly polarized light passes through the sample in an alternating fashion. The photomultiplier detector in the spectrometer produces a voltage proportional to the circular dichroism (the difference between the absorbance of left and right polarized light) of the resultant beam emerging from the sample. The circular dichroism of the sample is then compared with standard proteins to determine the differences in the secondary structure of the proteins. Uses of Circular dichroism spectroscopy The primary application for CD spectroscopy is the verification of the assumed secondary structure of the protein. This method allows the detection of percentages of α-helix and β-sheet in proteins base on their circular dichroism. Circular dichroism spectroscopy can also be used to monitor changes of secondary structure within a sample over time. This technique can also be used to compare two macromolecules to detect the differences in the structure of the molecules. It can also be used for the analysis of pharmaceutical products to ensure that they are still present in the folded active conformations....
Similar Free PDFs

Atomic Absorption Spectroscopy
- 271 Pages

Atomic Absorption Spectroscopy
- 9 Pages

Atomic Absorption Spectroscopy
- 1 Pages

Chem 1411 Atomic Spectroscopy
- 3 Pages

Spectroscopy Lecture Notes
- 52 Pages

The Atomic Theory notes
- 2 Pages

Civil Procedure Lecture Notes
- 23 Pages

Atomic theory - Lecture notes 2
- 7 Pages
Popular Institutions
- Tinajero National High School - Annex
- Politeknik Caltex Riau
- Yokohama City University
- SGT University
- University of Al-Qadisiyah
- Divine Word College of Vigan
- Techniek College Rotterdam
- Universidade de Santiago
- Universiti Teknologi MARA Cawangan Johor Kampus Pasir Gudang
- Poltekkes Kemenkes Yogyakarta
- Baguio City National High School
- Colegio san marcos
- preparatoria uno
- Centro de Bachillerato Tecnológico Industrial y de Servicios No. 107
- Dalian Maritime University
- Quang Trung Secondary School
- Colegio Tecnológico en Informática
- Corporación Regional de Educación Superior
- Grupo CEDVA
- Dar Al Uloom University
- Centro de Estudios Preuniversitarios de la Universidad Nacional de Ingeniería
- 上智大学
- Aakash International School, Nuna Majara
- San Felipe Neri Catholic School
- Kang Chiao International School - New Taipei City
- Misamis Occidental National High School
- Institución Educativa Escuela Normal Juan Ladrilleros
- Kolehiyo ng Pantukan
- Batanes State College
- Instituto Continental
- Sekolah Menengah Kejuruan Kesehatan Kaltara (Tarakan)
- Colegio de La Inmaculada Concepcion - Cebu