AAS Answers - Atomic Absorption Spectroscopy High level questions and graphing practice calibration PDF

| Title | AAS Answers - Atomic Absorption Spectroscopy High level questions and graphing practice calibration |
|---|---|
| Author | Ben Roberts |
| Course | HSC Chemistry - Year 12 |
| Institution | Higher School Certificate (New South Wales) |
| Pages | 1 |
| File Size | 27.1 KB |
| File Type | |
| Total Downloads | 84 |
| Total Views | 146 |
Summary
Atomic Absorption Spectroscopy High level questions and graphing practice calibration curve Answer...
Description
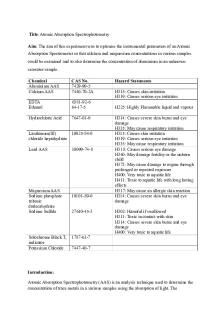
Analytical Techniques: AAS
SOLUTIONS
1. (a) Fixed amounts of energy (quanta) are required to promote electrons into higher energy levels. Only some wavelengths of light, corresponding to these quanta are absorbed. (b) The changes indicate movement of electrons from/to ground state to/from excited states. From/to gives an absorption spectrum; to/from an emission spectrum. (c) Yes, the wavelengths will be the same since energy is conserved. 2. (a) Ppm = mg L -1 (b) (1) ~4.5 ppm (2) ~6 ppm (3) ~8 ppm (c) ppm = mg L -1 so divide by 1000 to get g L -1 then divide by 10 to get g (100 mL) -1 (1) 0.00045 g (2) 0.0006 g (3) 0.0008 g (d) 0.00045 ×100 = 0.115% 0.39 0.0006 ×100 = 0.113% 0.53 0.0008 ×100 = 0.113% 0.71 average 0.114% = 0.11% to 2 s.f. So 0.11g Mg per 100g of sugar, ×104 gives 1.1×103 g per tonne ADDITIONAL 1 MARK FOR CORRECT SF 3. (a)
(1) ~450 ppm (2) ~450 mg L-1 (b) ÷100 (since there are 100 10mL samples in a litre) = 4.5 mg (c) During quantitative AAS the equipment is calibrated to only analyse one wavelength that is characteristic to (in this case) manganese ions. This wavelength will not be absorbed by other atoms and therefore they will not interfere with the results. ADDITIONAL 1 MARK for corect SF...
Similar Free PDFs

Atomic Absorption Spectroscopy
- 271 Pages

Atomic Absorption Spectroscopy
- 9 Pages

Atomic Absorption Spectroscopy
- 1 Pages

Chem 1411 Atomic Spectroscopy
- 3 Pages

AAS Questions
- 2 Pages

Spectroscopy Questions
- 116 Pages

Graphing practice for variables
- 3 Pages
Popular Institutions
- Tinajero National High School - Annex
- Politeknik Caltex Riau
- Yokohama City University
- SGT University
- University of Al-Qadisiyah
- Divine Word College of Vigan
- Techniek College Rotterdam
- Universidade de Santiago
- Universiti Teknologi MARA Cawangan Johor Kampus Pasir Gudang
- Poltekkes Kemenkes Yogyakarta
- Baguio City National High School
- Colegio san marcos
- preparatoria uno
- Centro de Bachillerato Tecnológico Industrial y de Servicios No. 107
- Dalian Maritime University
- Quang Trung Secondary School
- Colegio Tecnológico en Informática
- Corporación Regional de Educación Superior
- Grupo CEDVA
- Dar Al Uloom University
- Centro de Estudios Preuniversitarios de la Universidad Nacional de Ingeniería
- 上智大学
- Aakash International School, Nuna Majara
- San Felipe Neri Catholic School
- Kang Chiao International School - New Taipei City
- Misamis Occidental National High School
- Institución Educativa Escuela Normal Juan Ladrilleros
- Kolehiyo ng Pantukan
- Batanes State College
- Instituto Continental
- Sekolah Menengah Kejuruan Kesehatan Kaltara (Tarakan)
- Colegio de La Inmaculada Concepcion - Cebu