Kinetics Pre Lab PDF

| Title | Kinetics Pre Lab |
|---|---|
| Course | General Chemistry For Engineer |
| Institution | Montgomery College |
| Pages | 2 |
| File Size | 67.7 KB |
| File Type | |
| Total Downloads | 95 |
| Total Views | 143 |
Summary
This is the pre-lab assignment for the Kinetics group lab...
Description
A KINETICS EXPERIMENT REPORT SHEET Purpose: In this lab we are studying the chemical kinetics between iodine ion I- and the peroxydisulfate ion S2O82-. We do this lab to calculate the rate constant at the mentioned temperatures in the table and the activation energy (EA) for the reaction. We are adding catalyst (either Ag+ or Cu2+) and swirl in cold water to see at what temperature and time the reaction will happen and the solution changes color. We will repeat this for 3 times and get the average. Procedure: 1. Bring a 50 mL Erlenmeyer. You will need an “A” set of flasks and a “B” set of flasks We need to do each run at least twice. 2. Prepare the solutions indicated for your group in Table I. 3. For the room temperature runs, a reading of the lab temperature near your flasks will be fine. For the experiments at around 10 C, 30 C, and 40 C, the actual temperature of the water bath should be measured rather than being read off the of the water bath machines; for those experiments, go to step 4 after reading the following general procedure for mixing the solutions in the flasks: a. For each “A-B” set of flasks, one person should do the mixing, and one person run the stopwatch. Someone should record the change in color. Continue on stirring the solution. b. If you are adding a possible catalyst to a given data run, add one drop of that possible catalyst (either Ag+ or Cu2+) to one of the two flasks (A or B, it doesn't matter which) before mixing the two flasks together. The bottles of the two possible catalysts should be at the front of the room. c. When you're ready to mix the solutions, pour the solution from one flask into the other, then back and forth a few times. After the last transfer, gently swirl the contents of the flask containing both solutions continuously until the color change is observed. d. When the “observer" indicates the color of the solution has changed, stop the stopwatch. Record the time in seconds in your notebook.
e. Repeat the run with the same solutions. If the time you obtain differs by more than around 10 % from the first run, repeat the experiment a third time. Take the average of the two times that are closest to each other. Report this average on the group data table on the blackboard. 4: Additional information for water bath runs: a. Measure the temperature of the water in the bath. Do not assume the temperature is the value indicated on the controller display. b. After placing the solutions in the two flasks (A and B), place a loosely-fitting cork in each flask and put them in the holders in the water bath at the temperature assigned to your group. Make sure each flask is immersed in water at least halfway. Since each holder plate can accommodate up to 16, 50 mL flasks, to save time you may want to make up a duplicate set of flasks so the second (or third) run can be done immediately after the first. c. If you label the flasks with tape, please make sure the labels are high up (out of the water), and do not fall into the water bath; they can clog the machine. d. Allow the flasks to remain in the holders in the water bath for at least fifteen (15) minutes (equilibration period). About every three (3) minutes during this time, remove each flask from its holder, gently swir1 its contents while keeping the flask immersed in the water bath, then place it back in its holder. e. At the end of the equilibration period, uncork an “A” and a “B" flask, and when your timer says “go”, pour the contents of one flask into the other, then back and forth a few times as described in 3b. Keep the flasks immersed in the water bath as much as possible, especially after the final solution transfer, and swirl the immersed flask continuously until the color of the solution changes. Remember that the reaction may be significantly faster or slower than you expect based on your room temperature results due to the change in temperature! f. Repeat the run with the same solutions. Take the average of the two times. Report this average on the group data table on the blackboard. 5. Clean-up: Pour the solutions in the waste container' In the hood. Then clean all of the glassware and thermometers by rinsing them in the sinks....
Similar Free PDFs

Kinetics Pre Lab
- 2 Pages

Kinetics lab
- 3 Pages

Pre Lab 1 - pre lab
- 5 Pages

PRE LAB 8 - PRE LAB
- 5 Pages

Enzyme Kinetics Lab Simulation
- 4 Pages

Enzyme Kinetics Lab Report
- 14 Pages
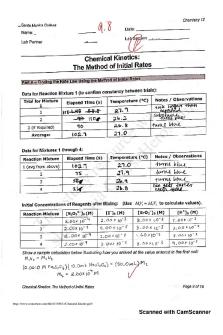
Chemical Kinetics lab
- 9 Pages

Chemical Kinetics - lab report
- 4 Pages

Kinetics Lab Report
- 8 Pages

Lab #9 Enzyme Kinetics
- 10 Pages

Lab Report - Enzyme Kinetics
- 4 Pages

Pre Lab #8 - pre lab 8
- 4 Pages

Chemistry Pre lab 1 - Pre Lab 1
- 3 Pages

Pre Lab 2 - Pre lab report 2
- 3 Pages
Popular Institutions
- Tinajero National High School - Annex
- Politeknik Caltex Riau
- Yokohama City University
- SGT University
- University of Al-Qadisiyah
- Divine Word College of Vigan
- Techniek College Rotterdam
- Universidade de Santiago
- Universiti Teknologi MARA Cawangan Johor Kampus Pasir Gudang
- Poltekkes Kemenkes Yogyakarta
- Baguio City National High School
- Colegio san marcos
- preparatoria uno
- Centro de Bachillerato Tecnológico Industrial y de Servicios No. 107
- Dalian Maritime University
- Quang Trung Secondary School
- Colegio Tecnológico en Informática
- Corporación Regional de Educación Superior
- Grupo CEDVA
- Dar Al Uloom University
- Centro de Estudios Preuniversitarios de la Universidad Nacional de Ingeniería
- 上智大学
- Aakash International School, Nuna Majara
- San Felipe Neri Catholic School
- Kang Chiao International School - New Taipei City
- Misamis Occidental National High School
- Institución Educativa Escuela Normal Juan Ladrilleros
- Kolehiyo ng Pantukan
- Batanes State College
- Instituto Continental
- Sekolah Menengah Kejuruan Kesehatan Kaltara (Tarakan)
- Colegio de La Inmaculada Concepcion - Cebu

