Lab Report for Qualitative Analysis of Group I Cations PDF

| Title | Lab Report for Qualitative Analysis of Group I Cations |
|---|---|
| Author | Oyinda A. |
| Course | General Inorganic Chemistry II |
| Institution | Howard Community College |
| Pages | 5 |
| File Size | 195.5 KB |
| File Type | |
| Total Downloads | 83 |
| Total Views | 137 |
Summary
Lab Report for Qualitative Analysis of Group I Cations...
Description
Adeyemo 1
Lab Report for Qualitative Analysis of Group I Cations Experiment #8 Chem 102-C502 October 29, 2020
Oyinda Adeyemo Lab Instructor: Professor Daria Rice Date: October 12, 2020
Adeyemo 2
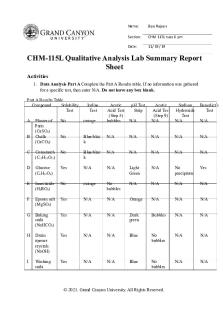
Purpose To become familiar with the chemical properties of the following dissolved cations: Ag+, Pb2+, Hg2+ Chemical Index Name of chemicals Silver (+1) cation Lead Mercury (1+) ion Hydrochloride acid Potassium Iodide Ammonia Nitric Acid
Molecular Formula Ag+ Pb+ Hg+ HCl KI NH3 HNO3
Molecular Weight 107.868 g/mol 207.2 g/mol 200.59 g/mol 36.46 g/mol 166.0028 g/mol 17.031 g/mol 63.01 g/mol
Equipment Centrifuge tubes, centrifuge, waste beaker, test tubes, litmus paper Procedure I – G1-1 Precipitation of Group I Cations
Measure out 10 drops of each of the three-test solution into a small centrifuge tube
Add 10 drops of 6M HCl, stir and centrifuge
Test for completeness of precipitation by adding 1 drop of 6M HCl to the supernatant
If supernatant turns cloudy, add two more drops of 6M HCl, stir and centrifuge
Repeat this process until no more precipitate forms
Decant the supernatant into a test tube and discard
Wash the precipitate by adding 5 drops of cold distilled water and stir
Centrifuge and decant the supernatants
Procedure II – G1-2 Separation and Identification of Pb2+
Adeyemo 3
Add 15 drops of hot water to the precipitate and place the test tube in a hot water bath
Stir Stan and heat solution for a minute
Centrifuge and decant the supernatant into a clean test tube
Repeat this procedure two more times and combine the supernatants into the same test tube
Save the precipitate for procedure G1-3
Add 3 drops of 1M of KI to into the test tube
A yellow or orange precipitate should be formed confirming that Pb2+ is present in the PbCl2 solution
Procedure III – G1-3 Separation and Identification Ag+ & Hg2+
At 10 drops of 6M NH3 to the precipitate from G1-2
Formation of a dark grey ppt indicates the presence of mercury
Centrifuge and decant the clear supernatant into a clean test tube
Add 20 drops of 6M HNO3 to decant
Stir the solution and test for its acidity
Continue to add a HNO3 dropwise until the solution is acidic and white cloudiness indicates the presence of Ag+
Testing the Unknown Measure 30 drops of the unknown solution Repeat steps G1-1 through G1-3 Identify the cations present in the known based on observation
Adeyemo 4
Data:
Conclusion
Adeyemo 5
The cations present in the unknown solution were Pb2+ and Hg2+. Ag+ wasn’t present in the experiment even after procedures were repeated. During the test to find Ag+, when HNO3 was added dropwise, the solution had 2 layers, the upper layer was clear while the lower layer had dark grey precipitate in it. This indicates that Hg2+ was still present in the solution. The known solutions had three Pb2+, Hg2+, Ag+ cations present in the solution. The purpose of the experiment was performed and understood. One error noticed during the experiment was during the decantation of the liquid to test for the Ag+. When the liquid was transferred, the mercury was all around the centrifuge tube which might have allowed part of the mercury to transfer into the tube. Sources of error noticed during this experiment will be avoided during future experiments. One equipment used during the experiment was the centrifuge. A centrifuge is a device that us used in the separation of fluids. In hospitals, a centrifuge can be used to separate red blood cells, plasmas and platelets. A daily life application of a centrifuge is a washing machine. After the clothes have been washed, the machine spins for a few minutes in order to separate the water from the clothes...
Similar Free PDFs

Qualitative Analysis of Cations
- 27 Pages

Qualitative lab report
- 11 Pages

Qualitative Analysis of Anions
- 11 Pages

Qualitative Analysis of Carbohydrate
- 13 Pages

QUANTITATIVE ANALYSIS GROUP REPORT
- 28 Pages

Lab Report 9 Group
- 3 Pages
Popular Institutions
- Tinajero National High School - Annex
- Politeknik Caltex Riau
- Yokohama City University
- SGT University
- University of Al-Qadisiyah
- Divine Word College of Vigan
- Techniek College Rotterdam
- Universidade de Santiago
- Universiti Teknologi MARA Cawangan Johor Kampus Pasir Gudang
- Poltekkes Kemenkes Yogyakarta
- Baguio City National High School
- Colegio san marcos
- preparatoria uno
- Centro de Bachillerato Tecnológico Industrial y de Servicios No. 107
- Dalian Maritime University
- Quang Trung Secondary School
- Colegio Tecnológico en Informática
- Corporación Regional de Educación Superior
- Grupo CEDVA
- Dar Al Uloom University
- Centro de Estudios Preuniversitarios de la Universidad Nacional de Ingeniería
- 上智大学
- Aakash International School, Nuna Majara
- San Felipe Neri Catholic School
- Kang Chiao International School - New Taipei City
- Misamis Occidental National High School
- Institución Educativa Escuela Normal Juan Ladrilleros
- Kolehiyo ng Pantukan
- Batanes State College
- Instituto Continental
- Sekolah Menengah Kejuruan Kesehatan Kaltara (Tarakan)
- Colegio de La Inmaculada Concepcion - Cebu