Qualitative Analysis summary report PDF

| Title | Qualitative Analysis summary report |
|---|---|
| Course | General Chemistry II - Lab |
| Institution | Grand Canyon University |
| Pages | 8 |
| File Size | 416.6 KB |
| File Type | |
| Total Downloads | 11 |
| Total Views | 141 |
Summary
Download Qualitative Analysis summary report PDF
Description
Name:
Biya Rajeev
Section:
CHM 115L tues 6 pm
Date:
11/ 19/ 19
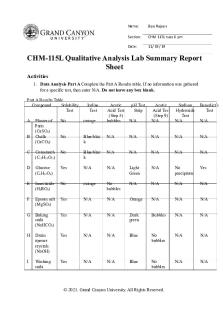
CHM-115L Qualitative Analysis Lab Summary Report Sheet Activities 1. Data Analysis Part A Complete the Part A Results table. If no information was gathered for a specific test, then enter N/A. Do not leave any box blank. Part A Results Table Compound Solubility Test A
Iodine Test
Acetic Acid Test (Step 5) bubbles
pH Test Strip
Sodium Hydroxide Test N/A
Benedict’s Test
N/A
Acetic Acid Test (Step 9) N/A
Plaster of Paris (CaSO4) Chalk (CaCO3)
No
orange
No
Blue/blac k
N/A
N/A
N/A
N/A
N/A
C
Cornstarch (C12H22O11)
No
Blue/blac k
N/A
N/A
N/A
N/A
N/A
D
Glucose (C6H12O6)
Yes
N/A
N/A
Light Green
N/A
No precipitate
Yes
E
Insecticide (H3BO3)
No
orange
No bubbles
N/A
N/A
N/A
N/A
F
Epsom salt (MgSO4)
Yes
N/A
N/A
Orange
N/A
N/A
N/A
G
Baking soda (NaHCO3)
Yes
N/A
N/A
Dark green
Bubbles
N/A
N/A
H
Drain opener crystals (NaOH)
Yes
N/A
N/A
Blue
No bubbles
N/A
N/A
I
Washing soda
Yes
N/A
N/A
Blue
No bubbles
N/A
N/A
B
© 2021. Grand Canyon University. All Rights Reserved.
N/A
Name:
Biya Rajeev
Section:
CHM 115L tues 6 pm
Date:
11/ 19/ 19
(Na2CO3) J
Table salt (NaCl)
Yes
N/A
N/A
Dark green
No bubbles
N/A
N/A
2. Data Analysis Part B a) Complete the following table using values from the notebook. If no information was gathered for a specific test, then enter N/A. Do not leave any box blank. Compound
Solubility Test
Iodine Test
Acetic Acid Test (Step 5)
pH Test Strip
Acetic Acid Test (Step 9)
Sodium Hydroxide Test
Benedict’s Test
Unknown (1) Unknown (2)
No
Orange
No bubbles
N/A
N/A
N/A
N/A
Unknown (3)
Yes
N/A
N/A
Orange
N/A
N/A
N/A
Unknown (4) Unknown (5)
Yes
N/A
N/A
Dark green
Bubbles
N/A
N/A
b) Identify the unknown compounds Unknown # 1
Identification NA
2
Insecticide
3
Epsom salt
4
Baking soda
5
NA
© 2021. Grand Canyon University. All Rights Reserved.
Name:
Biya Rajeev
Section:
CHM 115L tues 6 pm
Date:
11/ 19/ 19
3. Flowcharts a) Insert here the completed Flowchart A1.
A,B,C,D,E,F,G,H,I,J
A,B,C,E
D,F,G,H,I,J
E
C,B
A,E
A
© 2021. Grand Canyon University. All Rights Reserved.
Name:
Biya Rajeev
Section:
CHM 115L tues 6 pm
Date:
11/ 19/ 19
b) Insert here the completed Flowchart A2.
D,F,G,H,I,J
I,H
J,G
F
D
G D
I,H
D
© 2021. Grand Canyon University. All Rights Reserved.
Name:
Biya Rajeev
Section:
CHM 115L tues 6 pm
Date:
11/ 19/ 19
4. Post-Lab Questions 1. Describe the difference between quantitative and qualitative results. Give an example of each. Quantitative results involve numbers and accurate measurements while qualitative results are more subjective and connected to our senses. A quantitative measurement would be that a pencil is 5 ins tall. A Qualitative measurement would be that a pencil is yellow and lean. 2. Of the substances that produced bubbles upon the addition of acetic acid, what do they have in common? What gas was produced? Both substances have Oxygen atoms, this allows them to form gases such as carbon dioxide very easily. The gas that was produced was Carbon Dioxide. 3. Based on the test results, list each compound and properties determined about it through the different tests performed. Some of these can include solubility, polarity, acidity/basicity, and formation of gas. A – Plaster of Paris: -
Not soluble
-
Iodine test- no starch
-
Acetic Acid test – presence of carbonate/bicarbonate detected
-
pH test – NA
-
Sodium hydroxide test – NA
-
Benedict’s test – NA
B – Chalk: -
Not soluble
-
Iodine test – starch
-
Acetic Acid test – NA
-
pH test – NA
-
Sodium hydroxide test – NA
-
Benedict’s test – NA
C – cornstarch: -
Not soluble
-
Iodine test – starch
© 2021. Grand Canyon University. All Rights Reserved.
-
Acetic Acid test – NA
-
pH test – NA
-
Sodium hydroxide test – NA
-
Benedict’s test – NA
Name:
Biya Rajeev
Section:
CHM 115L tues 6 pm
Date:
11/ 19/ 19
D – glucose: -
Soluble
-
Iodine test – NA
-
Acetic Acid test – NA
-
pH test – slightly acidic
-
Sodium hydroxide test – no presence of cations detected
-
Benedict’s test – presence of Glucose detected
E – Insecticide: -
Not soluble
-
Iodine test – no starch
-
Acetic Acid test – no presence of carbonate/bicarbonate
-
pH test – NA
-
Sodium hydroxide test – NA
-
Benedict’s test – NA
F – Epson salt: -
Soluble
-
Iodine test – NA
-
Acetic Acid test – NA
-
pH test – very acidic
-
Sodium hydroxide test – NA
-
Benedict’s test – NA
G – baking soda: -
Soluble
-
Iodine test – NA
-
Acetic Acid test – presence of carbonate/bicarbonate detected
-
pH test – slightly alkaline
© 2021. Grand Canyon University. All Rights Reserved.
-
Sodium hydroxide test – NA
-
Benedict’s test - NA
Name:
Biya Rajeev
Section:
CHM 115L tues 6 pm
Date:
11/ 19/ 19
H – drain opener: -
Soluble
-
Iodine test – NA
-
Acetic Acid test – NA
-
pH test – alkaline
-
Sodium hydroxide test – NA
-
Benedict’s test – NA
I – washing soda: -
Soluble
-
Iodine test – NA
-
Acetic Acid test – no presence of carbonate/bicarbonate detected
-
pH test – alkaline
-
Sodium hydroxide test – NA
-
Benedict’s test – NA
J – table salt: -
Soluble
-
Iodine test – NA
-
pH test - Neutral
-
Acetic Acid test – no presence of carbonate/bicarbonate detected
-
Sodium hydroxide test – NA
-
Benedict’s test – NA
4. What was the most difficult part about identifying your unknowns? What was the easiest? A specific part didn’t stand out to be difficult, every test ran pretty smoothly and the flow chart made everything very clear, The flowchart was probably the most helpful when testing the unknown. 5. Copies of Notebook Pages
© 2021. Grand Canyon University. All Rights Reserved.
Name:
Biya Rajeev
Section:
CHM 115L tues 6 pm
Date:
11/ 19/ 19
Submit the copies of the notebook pages recorded for this lab. Make sure that the notebook pages meet the criteria provided by the instructor.
© 2021. Grand Canyon University. All Rights Reserved....
Similar Free PDFs

Qualitative Analysis of Anions
- 11 Pages

Exam Qualitative analysis sample
- 4 Pages

Qualitative lab report
- 11 Pages

Qualitative Analysis of Carbohydrate
- 13 Pages

Task 1 - Qualitative analysis
- 4 Pages

Qualitative Structural Analysis
- 18 Pages

Qualitative Analysis - Beams
- 15 Pages

Qualitative Analysis of Cations
- 27 Pages

Qualitative Analysis-Sp21
- 6 Pages

5. Qualitative Movement Analysis
- 1 Pages
Popular Institutions
- Tinajero National High School - Annex
- Politeknik Caltex Riau
- Yokohama City University
- SGT University
- University of Al-Qadisiyah
- Divine Word College of Vigan
- Techniek College Rotterdam
- Universidade de Santiago
- Universiti Teknologi MARA Cawangan Johor Kampus Pasir Gudang
- Poltekkes Kemenkes Yogyakarta
- Baguio City National High School
- Colegio san marcos
- preparatoria uno
- Centro de Bachillerato Tecnológico Industrial y de Servicios No. 107
- Dalian Maritime University
- Quang Trung Secondary School
- Colegio Tecnológico en Informática
- Corporación Regional de Educación Superior
- Grupo CEDVA
- Dar Al Uloom University
- Centro de Estudios Preuniversitarios de la Universidad Nacional de Ingeniería
- 上智大学
- Aakash International School, Nuna Majara
- San Felipe Neri Catholic School
- Kang Chiao International School - New Taipei City
- Misamis Occidental National High School
- Institución Educativa Escuela Normal Juan Ladrilleros
- Kolehiyo ng Pantukan
- Batanes State College
- Instituto Continental
- Sekolah Menengah Kejuruan Kesehatan Kaltara (Tarakan)
- Colegio de La Inmaculada Concepcion - Cebu