Chapter 5-02 - Pearson - Homework PDF

| Title | Chapter 5-02 - Pearson - Homework |
|---|---|
| Course | Biological Principles For Non-Majors |
| Institution | Broward College |
| Pages | 16 |
| File Size | 838.1 KB |
| File Type | |
| Total Downloads | 42 |
| Total Views | 151 |
Summary
Pearson - Homework...
Description
Chapter 5-02 Due: 10:59pm on Tuesday, November 30, 2021 You will receive no credit for items you complete after the assignment is due. Grading Policy
MP3 Tutor Session: Basic Energy Concepts Click the image below to listen to the MP3 Tutor Session. You can also download the MP3 or view the text of the tutor session to read while you are listening. Estimated time: 8 minutes, 47 seconds. After you have listened to the tutor session, answer the questions.
Part A Energy is conserved. This means that in any system, _____. ANSWER:
high-quality energy input equals high-quality energy output total energy input equals total energy output light energy is released to replace the original input of solar energy energy is constantly recycled
Correct
Part B Kinetic energy is energy in motion. Potential energy is _____ energy. ANSWER:
heat solar electromagnetic stored
Correct
Part C Which of the following is highest in chemical energy? ANSWER: one molecule of H2 one molecule of glucose one molecule of CO2 one molecule of ATP
Correct
Part D In cellular respiration, most energy is released and transferred to ATP when _____. ANSWER:
high-energy electrons "fall" to lower energy levels low-energy electrons are "raised" to higher energy levels low-energy C-H bonds in glucose are broken high-energy C-C and C-H bonds in glucose are broken
Correct
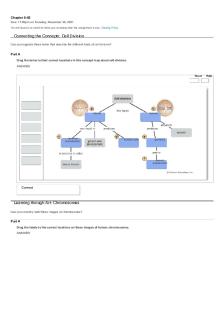
Connecting the Concepts: Energy Can you complete this concept map that reviews the basic concepts of energy?
Part A Drag the terms to the appropriate locations on the concept map. ANSWER:
Reset
kinetic
potential
motion
chemical energy entropy
first law of thermodynamics
Correct
Figure Walkthrough: Energy Transformations in a Car and in a Cell Watch this video and then answer the questions.
second law of thermodynamics
heat
Help
Part A The figure shows how cellular respiration is similar to combustion in a car. Can you identify the inputs, outputs, and processes? Drag the labels to their appropriate locations on the figure. Blue labels represent processes, and pink labels represent inputs and outputs. Pink labels may be used more than once. ANSWER:
Reset
Heat energy
Heat energy
Combustion
Glucose
Carbon dioxide
Carbon dioxide Water
Oxygen
Water
Oxygen Heat energy Cellular respiration
ATP
Carbon dioxide Glucose ATP
Oxygen
Correct
Part B How are combustion and cellular respiration different? ANSWER:
Combustion produces heat, but cellular respiration does not. Cellular respiration requires oxygen, but combustion does not. Cellular respiration produces carbon dioxide and water, but combustion does not. Cellular respiration breaks down sugar, and combustion breaks down octane.
Water
Help
Correct The energy-rich molecules that are broken down are different in combustion and cellular respiration, but both processes require oxygen and produce carbon dioxide, water, and heat. Energy in cellular respiration is also captured to make ATP.
Part C What is the function of ATP? ANSWER:
It is a waste product of cellular respiration. It is a form of heat. It provides energy for cellular work. It stores kinetic energy.
Correct The potential energy in ATP can be used in a variety of ways, such as powering movement, synthesis of molecules, and transport of materials.
Visualizing the Data: Consuming and Burning Calories Calories are a standard unit of energy. They describe both how much energy is contained in food, and how much energy is burned by bodily activities. Explore the interactive figure to compare different foods and different activities to see how many calories each contains or burns. Then answer the questions.
Part A Of the listed foods, which one has the fewest calories? ANSWER:
fries garden salad with ranch dressing egg orange
Correct Of the foods listed in this figure, an orange contains the fewest calories.
Part B If you ate two slices of pepperoni pizza and wish to burn off all the calories, how long would you have to ride your bike to achieve that? ANSWER:
28 minutes 45 minutes 56 minutes 280 minutes
Correct A single slice of pepperoni pizza contains 280 Calories. To burn 280 Calories on your bike, you would have to ride for 28 minutes. Therefore, you would have to ride for twice that long—56 minutes—to burn off the calories from two slices of pepperoni pizza.
Part C Imagine you eat an extra serving of fries every day for a week but don’t increase your activity level. Knowing that an extra 3,500 Calories results in a weight gain of one pound, how would this additional food affect your weight? ANSWER:
3,500 pounds 1 pound 3.50 pounds It cannot be determined from the information given.
Correct Each serving of fries contains 500 Calories. Consuming an extra serving for 7 days = 500
7 = 3,500 Calories = 1 extra pound.
Part D If you ate one cheeseburger and wish to burn off all the calories, which of the listed activities would accomplish that the fastest? ANSWER:
jumping rope running walking swimming
Correct Of the activities listed in the figure, jumping rope burns calories the fastest, taking 30 minutes to burn off the calories in a cheeseburger.
Part E Which of the following foods is correctly matched with an activity that would burn all the calories it contains? Select all that apply. ANSWER: 2 oranges and 16 minutes of walking 1 cheeseburger and 66 minutes of playing baseball 3 slices of pepperoni pizza and 75 minutes of running 2 oranges and 8 minutes of walking 1 slice of pepperoni pizza and 75 minutes of running
Correct By comparing different foods and activities, you can get a sense of how long you’d have to maintain each type of exercise to burn off the equivalent calories in each food.
Activity: Chemical Reactions and ATP
Click here to complete this activity. Then answer the questions.
Part A In this reaction _____.
ANSWER:
the products have been rearranged to form reactants AC is a reactant CD is a product the products have less potential energy than the reactants entropy has decreased
Correct This is what is shown by the graph.
Part B In this reaction _____.
ANSWER:
heat has been released to the environment disorder has decreased the chemical energy of the products is greater than that of the reactants entropy has decreased the kinetic energy of the reactants is less than that of the products
Correct The potential energy of the products is less than that of the reactants.
Part C The reaction A --> B + C + heat is released in a(n) _____ reaction. ANSWER:
dehydration synthesis exergonic endergonic exchange anabolic
Correct Energy has been released.
Part D A(n) _____ reaction occurs spontaneously. ANSWER: kinetic endergonic exergonic chemical anabolic
Correct In exergonic reactions the products have less potential energy than the reactants.
Part E Which of these reactions requires a net input of energy from its surroundings? ANSWER:
exergonic hydrolysis ATP --> ADP + P catabolic endergonic
Correct The products of endergonic reactions have more potential energy than the reactants.
Part F In cells, what is usually the immediate source of energy for an endergonic reaction? ANSWER:
sugar as spontaneous reactions, endergonic reactions do not need an addition of energy ADP glucose ATP
Correct The hydrolysis of ATP provides the energy needed for an endergonic reaction.
Part G The reaction ADP + P --> ATP is a(n) _____ reaction. ANSWER:
hydrolysis exergonic chemical endergonic spontaneous
Correct Energy has been acquired from the surroundings.
Part H The energy for an endergonic reaction comes from a(n) _____ reaction. ANSWER: anabolic synthesis exergonic ADP + P --> ATP glucose + glucose --> maltose
Correct The energy released by an exergonic reaction can be used to drive an endergonic reaction.
Part I What is the fate of the phosphate group that is removed when ATP is converted to ADP? ANSWER:
It is used to convert an ATP into an AQP. It is acquired by a reactant in an endergonic reaction. It is broken down into one phosphorus and four oxygen atoms. It is acquired by a reactant in an exergonic reaction. It is acquired by a reactant in a spontaneous reaction.
Correct By acquiring the phosphate group the reactant acquires energy.
Part J This graph illustrates a(n) _____ reaction.
ANSWER:
endergonic hydrolysis exergonic spontaneous catabolic
Correct The products contain more potential energy than the reactants.
Part K Select the INCORRECT association. ANSWER: exergonic ... spontaneous enzyme ... protein kinetic energy ... motion potential energy ... positional energy exergonic ... uphill
Correct Exergonic reactions release energy.
Part L What is energy coupling? ANSWER:
a description of the energetic relationship between the reactants and products in an exergonic reaction a barrier to the initiation of a reaction the hydrolysis of ATP to ADP + P the use of an enzyme to reduce EA the use of energy released from an exergonic reaction to drive an endergonic reaction
Correct This is energy coupling.
Activity: How Enzymes Work
Click here to complete this activity. Then answer the questions.
Part A Most enzymes are _____. ANSWER:
proteins lipids carbohydrates minerals nucleic acids
Correct Most enzymes are proteins.
Part B Enzymes work by _____. ANSWER:
increasing the potential energy difference between reactant and product reducing activation energy decreasing the potential energy difference between reactant and product adding energy to a reaction adding a phosphate group to a reactant
Correct Enzymes work by reducing the energy of activation.
Part C An enzyme _____. ANSWER:
increases the the activation energy of a reaction is a source of energy for endergonic reactions can bind to nearly any molecule is a inorganic catalyst is an organic catalyst
Correct Enzymes are proteins that behave as catalysts.
Part D What name is given to the reactants in an enzymatically catalyzed reaction? ANSWER:
products substrate active sites reactors
Correct This is the name given to the reactants in an enzymatically catalyzed reaction.
Part E As a result of its involvement in a reaction, an enzyme _____. ANSWER:
loses a phosphate group permanently alters its shape. is used up loses energy is unchanged
Correct Enzymes are not changed as a result of their participation in a reaction.
Part F What is the correct label for "A"?
ANSWER:
substrate energy ATP uphill enzyme energy energy of activation
Correct The energy of activation must be overcome in order for a reaction to proceed.
Building Vocabulary: Enzymes Can you place each word in the appropriate sentence?
Part A Drag the terms on the left to the appropriate blanks on the right to complete the sentences. ANSWER:
Reset
Help
The specific location within an enzyme molecule where the substrate binds is called the active site .
Enzymes speed up chemical reactions by lowering the activation energy , which allows the reaction to proceed much more quickly. The induced fit
between an active site and its substrate often strains bonds and helps the reaction
proceed. During an enzymatic reaction, a molecule of substrate
binds to the enzyme and is broken down into
one or more molecules of product , which are released. Lactose takes years to break down on its own. But if exposed to the protein lactase, the reaction proceeds very quickly, while lactase itself remains unchanged. Lactase is an example of a(n) enzyme .
A(n) inhibitor
is a molecule that can bind to an enzyme and prevent the enzyme from working.
An enzyme is considered a(n) catalyst
because it speeds up a chemical reaction without being
used up. High temperatures or changes in pH can denature
an enzyme, causing it to lose its shape and
biological activity.
Correct
Interpreting Data: Enzymes and Chemical Reactions This graph illustrates the course of a chemical reaction with and without an enzyme. While the products and reactants are the same, the presence of an enzyme changes the amount of energy required for the reaction to begin.
Part A - Interpreting the graph Which curve shows the course of the reaction in the presence of an enzyme--the black curve or the red curve? Which line represents the activation energy for that reaction-a, b, or c? ANSWER:
black curve; line a black curve; line b black curve; line c red curve; line a red curve; line b red curve; line c
Correct
Part B - Thinking critically Use the graph and your knowledge of enzymes to identify the three true statements about enzymes. ANSWER:
Reactants cannot convert to products without an initial input of energy to start the reaction. Only reactions that are controlled by enzymes require activation energy. Chemical reactions cannot occur without enzymes. Enzymes lower the overall energy input needed for a reaction to occur. By binding to reactant molecules, enzymes make it easier for the bonds in the molecules to break apart.
Correct For a chemical reaction to begin, chemical bonds in the reactant molecules must be broken. This process requires that the molecules absorb energy from their surroundings. The energy that must be invested to start a reaction is called activation energy because it activates the reactants and triggers the chemical reaction. Enzymes enable metabolism to occur by reducing the amount of activation energy required to break the bonds of reactant molecules.
Everyday Biology: How to Keep Your Cool Watch the Everyday Biology video, read the accompanying essay, and then answer the questions below.
On Tuesday July 31, 2001, the Minnesota Vikings football team was on the field for another day of practice at their summer training camp. The temperature rose to 91°F, but with the high humidity the heat index was nearly 110°F. Korey Stringer, a 27-year-old offensive tackle on the team, complained of breathing problems and began vomiting. He was rushed to the hospital with a core body temperature of 108°F. He died early Wednesday morning, leaving behind a wife and a 3-year-old son. Korey Stringer died of heat stroke, a condition where the body temperature exceeds 104°F, causing symptoms such as nausea, vomiting, fatigue, and rapid pulse. If not treated in time, heat stroke can lead to death as the body’s organs shut down. Stringer was a professional football player with years of experience on the field. He had been a first round draft pick in 1995 after a standout career at Ohio State University, and was a veteran of hard work and long days of practice on the field. However, even the most experienced athletes are no match for the damage that heat can cause at the molecular level. Unfortunately, we might not even realize we are dangerously close to experiencing heat stroke until it is too late. Heat stroke occurs when body temperature rises to the point where the body’s enzymes lose their three-dimensional shape. These include the enzymes that regulate the heart, the nervous system, and all the other organs and systems in the body. Because the active site of an enzyme must fit over a specific substrate, the three-dimensional shape of the enzyme is critical to its function. If the three-dimensional shape is changed at the molecular level, then the enzyme doesn’t do its job. This change in the shape of the enzyme, and its loss of proper functioning, is called denaturation. Sometimes denaturation is temporary – with some enzymes, you can raise the temperature slightly to denature the enzyme, then cool it back down to restore it to its original shape with no ill effects. But sometimes denaturation is permanent. This is what happens when the proteins in an egg white change from being runny and transparent in a raw egg to being firm and opaque in a fried egg – once you fry an egg, there’s no turning back! When his body temperature climbed above 104°F, Korey Stringer’s enzymes began denaturing, and his organs began to fail. Whether the denaturing of the enzymes was temporary or permanent didn’t matter, as his body temperature stayed too high for too long. Enzymes in the human body work best at normal body temperature, which is around 98.6°F. The body can tolerate some deviation from this temperature without causing any harm. For example, a slight fever can raise the body temperature enough to slow the growth and spread of bacteria that may be causing an infection, giving the body’s immune system time to kill the bacteria. A slight drop in body temperature can affect a person’s ability to move or think clearly, but there are no lasting problems if the body can be quickly warmed back up to its normal temperature. However, extreme hypothermia (a drop in body temperature) or hyperthermia (a rise in body temperature) that lasts too long can denature enzymes to the point where the person can no longer survive. In an effort to prevent more deaths from heat stroke, the researchers at Stanford University developed the cooling system shown in the video. Holding onto the cold metal cone works well because there are so many blood vessels in the palm of the hand. The transfer of heat from the hand to the circulating cold water in the metal cone helps lower the body’s temperature quickly and efficiently. But you might wonder – what is so special about the glove? Why not just stick your hand in ice water to cool down? The problem with that approach is that sticking your hand in ice water causes a physiological response to being cold, where the blood vessels in the hand constrict, reducing blood flow, in order to hold more heat in the body’s core. So there is one more feature of Stanford’s glove system, which was not discussed in the video, but which was the part of the glove that you see being tightened around the wrist. Once the wrist piece is tightened, the gloves have an airtight fit. With the airtight fit, air can be sucked out of the glove to create a vacuum within the glove. The vacuum within the glove helps keep the blood vessels in the hand wide open, maintaining blood circulation in the hand, facilitating the transfer of heat to the metal cone and then to the circulating cold water supply inside the cone. Today, athletes in a variety of sports are using these gloves and similar devices. Someday, devices like these may become commonplace where people who are at risk of heat stroke work and play. Perhaps if they had been available when Korey Stringer was practicing, he might still be alive.
Part A Why does the reporter have a tube up his nose? ANSWER:
to recycle the water he loses during the test to replace the salts and other ions lost during the test to accurately measure core temperature as a feeding tube to replace lost nutrients
Correct
Part B
The r...
Similar Free PDFs
Chapter 8-02 - Pearson - Homework
- 22 Pages

Chapter 8-01 - Pearson - Homework
- 15 Pages

Chapter 3-02 - Pearson - Homework
- 17 Pages

Chapter 7-02 - Pearson - Homework
- 17 Pages

Chapter 9-01 - Pearson Homework
- 17 Pages
Chapter 5-01 - Pearson - Homework
- 13 Pages

Chapter 5-02 - Pearson - Homework
- 16 Pages

Chapter 13-01 - Pearson Homework
- 14 Pages

Chapter 7-01 - Pearson - Homework
- 18 Pages

Chapter 9-02 - Pearson Homework
- 12 Pages

Chapter 10-03 - Pearson Homework
- 8 Pages

Chapter 10-02 - Pearson Homework
- 12 Pages

Chapter 6-01 - Pearson - Homework
- 14 Pages

Chapter 10-01 - Pearson Homework
- 20 Pages

P141 - Pearson
- 1 Pages
Popular Institutions
- Tinajero National High School - Annex
- Politeknik Caltex Riau
- Yokohama City University
- SGT University
- University of Al-Qadisiyah
- Divine Word College of Vigan
- Techniek College Rotterdam
- Universidade de Santiago
- Universiti Teknologi MARA Cawangan Johor Kampus Pasir Gudang
- Poltekkes Kemenkes Yogyakarta
- Baguio City National High School
- Colegio san marcos
- preparatoria uno
- Centro de Bachillerato Tecnológico Industrial y de Servicios No. 107
- Dalian Maritime University
- Quang Trung Secondary School
- Colegio Tecnológico en Informática
- Corporación Regional de Educación Superior
- Grupo CEDVA
- Dar Al Uloom University
- Centro de Estudios Preuniversitarios de la Universidad Nacional de Ingeniería
- 上智大学
- Aakash International School, Nuna Majara
- San Felipe Neri Catholic School
- Kang Chiao International School - New Taipei City
- Misamis Occidental National High School
- Institución Educativa Escuela Normal Juan Ladrilleros
- Kolehiyo ng Pantukan
- Batanes State College
- Instituto Continental
- Sekolah Menengah Kejuruan Kesehatan Kaltara (Tarakan)
- Colegio de La Inmaculada Concepcion - Cebu
