Week 2 Assignment SC1040 PDF
| Title | Week 2 Assignment SC1040 |
|---|---|
| Author | Aaron Burleson |
| Course | Biology |
| Institution | Ultimate Medical Academy |
| Pages | 5 |
| File Size | 337 KB |
| File Type | |
| Total Downloads | 37 |
| Total Views | 155 |
Summary
UMA BIOLOGY...
Description
SC1040: Week 2 Assignment
How does Chemistry apply to Biol Biology? ogy? This assignment helps you apply your knowledge from this week’s modules and readings. Understanding the basics of chemistry is important to your healthcare career; every living organism is a chemical system at its basic foundation. This week’s concepts are also concerned with understanding the chemistry you encounter in your daily life.
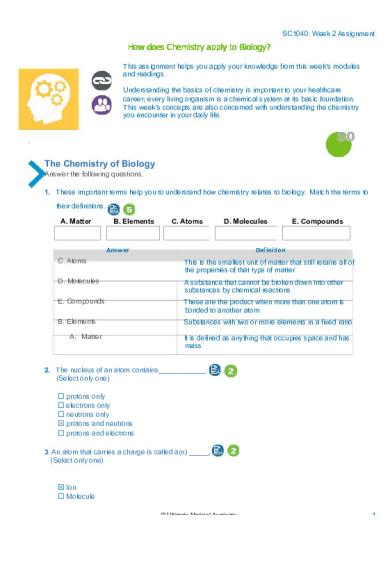
50 The Chemistry of Biology Answer the following questions. 1. These important terms help you to understand how chemistry relates to biology. Match the terms to their definitions. A. Matter
B. Elements
C. Atoms
D. Molecules
Answer
E. Compounds
Definition
C. Atoms
This is the smallest unit of matter that still retains all of the properties of that type of matter
D. Molecules
A substance that cannot be broken down into other substances by chemical reactions
E. Compounds
These are the product when more than one atom is bonded to another atom
B. Elements
Substances with two or more elements in a fixed ratio
A. Matter
It is defined as anything that occupies space and has mass
2. The nucleus of an atom contains____________. (Select only one) ☐ protons only ☐ electrons only ☐ neutrons only ☒ protons and neutrons ☐ protons and electrons 3. An atom that carries a charge is called a(n) _____. (Select only one)
☒ Ion ☐ Molecule © Ultimate Medical Academy
1
☐ Compound ☐ Element ☐ Microelement 4. When atoms share electrons unequally, the bond formed is a(n)__________bond. (Select only one) ☐ Double ☐ Hydrogen ☐ Ionic ☒ Polar covalent ☐ Non-polar covalent 5. A(n)__________ is a type of chemical bond in which a strong mutual attraction forms between ions of opposite charge. (Select only one) ☐ Hydrogen bond ☐ Nonpolar bond ☐ Polar bond ☐ Covalent bond ☒ Ionic bond 6. Life as we know it could not exist without chemical reactions. For example, water is created by a chemical reaction between two highly volatile elements: oxygen and hydrogen.
Oxygen = a highly reactive gas with a boiling point of minus 297.3 degrees Hydrogen = odorless, colorless, and tasteless (so it’s undetectable to human senses) but highly flammable and used in jet fuel Identify another chemical reaction that is important to your daily life. (Hint: THIS SITE is a good resource.) Photosynthesis is important so that plants live and grow and are able to give off the oxygen we need to be able to live.
7. A __________ is an organic molecule that consists of C, H, O atoms in a ratio of 1:2:1. ☒ carbohydrate ☐ protein
© Ultimate Medical Academy
2
8. Carbon can bond with up to_________ other atoms. (Select only one)
☐1 ☐2 ☐3 ☒4 ☐5
Water and Hydrogen Bonds Chemical reactions between molecules drive the biological processes of life. There are many different types of bonds and each one is formed differently. Answer the following questions. 9. Which type of chemical bond holds the atoms together within a molecule of water? Covalent Bonds 10. Water is referred to as a polar molecule. Describe the characteristics of a polar molecule. The characteristics of a polar molecule include one side that is negatively charged and one side that is positively charged, melting and boiling points, and surface tension. 11. Any substance dissolved in water is called a __________.
(Select only one) ☐ Concentration ☐ Salt ☒ Solute ☐ solution 12. Water is considered the “universal solvent” because it dissolves more substances than any other liquid. Wherever water flows, it carries dissolved chemicals, minerals, and nutrients. Watch this VIDEO and then describe one reason why the solvent property of water is so important for living organisms(nature). Water is important to humans especially, because it allows nutrients and sugars to be carried through our bodies, which is very important for survival. 13. Water is an essential compound that is balanced. Please watch this VIDEO about the importance of water to your body and then answer the following questions. a. Explain why water is important for maintaining good health. Water is important because it maintains every function in your body. From your skin to your brain, water is essential. © Ultimate Medical Academy
3
b. Describe the risks linked to dehydration. Cognitive impairment, low blood pressure, depression, dry skin, and low energy.
The pH Scale The pH scale is a measure of acidity and runs from 0 (most acidic) to 14 (most basic).
14. The pH of our blood is strictly maintained between 7.3 and 7.5 by a ______. (Select only one) ☐ Acid ☐ Base ☐ Proton ☒ Buffer ☐ Salt
Organic Molecules 15. Organic molecules are often referred to as the “molecules of life.” Watch this VIDEO about organic molecules and identify each one by matching it to its function. Organic Molecule Carbohydrates___ B Lipids___ C Protein___ A
Function a. enzymes during photosynthesis, antibodies for fighting infection b. responsible for producing different kinds of sugar molecules c. stored as fat, Vitamins A&D
© Ultimate Medical Academy
4
Nucleic Acids Nucleic acids are organic molecules essential for all living things. Watching the following VIDEO and answer the following questions.
16. A Nucleotide has 6 carbon sugars. ☐ True ☒ False 17. The nucleotide __________ serves as the energy carrier molecule in cells. (Select only one)
☒ ATP ☐ DNA 18. __________ contains two nucleotide chains twisted into a helix. (Select only one)
☒ DNA ☐ RNA
Protein Functions Click HERE to read this brief video about the 6 primary functions of proteins.
19. List the six (6) functions of proteins. a. Energy b. Hormones c. Repair and Maintanence d. Enzymes e. Transportation and storage of molecules f.
Builds Antibodies.
© Ultimate Medical Academy
5...
Similar Free PDFs

Week 2 Assignment SC1040
- 5 Pages

Week 3 Assignment SC1040
- 6 Pages

Week 5 Assignment SC1040
- 6 Pages

Week 4 - Assignment 2
- 15 Pages

Week 2 Assignment
- 2 Pages

Week 2assignment - Assignment 2
- 6 Pages

Week 2 - Assignment
- 3 Pages

Week 2 - Assignment Linux
- 13 Pages

EN1150 Week 2 Assignment
- 6 Pages

BUS600 Week 2 Assignment
- 6 Pages

Week 2 assignment pol201
- 2 Pages

Week 5 assignment 2
- 15 Pages

Assignment-Week 2
- 2 Pages

Week 2 Assignment
- 7 Pages

Ece313 week 2 assignment
- 6 Pages
Popular Institutions
- Tinajero National High School - Annex
- Politeknik Caltex Riau
- Yokohama City University
- SGT University
- University of Al-Qadisiyah
- Divine Word College of Vigan
- Techniek College Rotterdam
- Universidade de Santiago
- Universiti Teknologi MARA Cawangan Johor Kampus Pasir Gudang
- Poltekkes Kemenkes Yogyakarta
- Baguio City National High School
- Colegio san marcos
- preparatoria uno
- Centro de Bachillerato Tecnológico Industrial y de Servicios No. 107
- Dalian Maritime University
- Quang Trung Secondary School
- Colegio Tecnológico en Informática
- Corporación Regional de Educación Superior
- Grupo CEDVA
- Dar Al Uloom University
- Centro de Estudios Preuniversitarios de la Universidad Nacional de Ingeniería
- 上智大学
- Aakash International School, Nuna Majara
- San Felipe Neri Catholic School
- Kang Chiao International School - New Taipei City
- Misamis Occidental National High School
- Institución Educativa Escuela Normal Juan Ladrilleros
- Kolehiyo ng Pantukan
- Batanes State College
- Instituto Continental
- Sekolah Menengah Kejuruan Kesehatan Kaltara (Tarakan)
- Colegio de La Inmaculada Concepcion - Cebu
