8. Introduction to membrane transport PDF

| Title | 8. Introduction to membrane transport |
|---|---|
| Author | Aimee White |
| Course | Biochemistry |
| Institution | University of East Anglia |
| Pages | 4 |
| File Size | 402.4 KB |
| File Type | |
| Total Downloads | 48 |
| Total Views | 172 |
Summary
Lecture notes from Biochemistry, Year 2020/21....
Description
BIOLOGICAL MEMBRANES: PRINCIPLES OF MEMBRANE TRANSPORT Every cell needs to interact with its environment to survive. The roles of membrane transport are: -
Regulate cell volume Maintain cell pH and ionic concentration Generate ionic gradients Concentrate metabolic fuels Extrude toxins
This transport is needed to uptake and concentrate metabolic fuels from the environment; glucose is stored or used during respiration, either way maintaining the concentration gradient. It is also needed to remove toxins and waste from the cell. Plus, it maintains cellular ion concentration/gradients and pH
Maintains pH optimum for enzymatic activity and the cell at optimal pH proton gradient for ATP synthesis (proton motive force). The enzymes involved are NADH dehydrogenase, succinate dehydrogenase, cytochrome bc1 complex and cytochrome c oxidase.
1. Laws of thermodynamics. First law: Law of conservation of energy- The energy cannot be created or destroyed, it can only be redistributed or changed from one form to another. Second law: The entropy of any isolated system not in thermal equilibrium always increases. During diffusion, entropy will increase over time to a maximum at equilibrium and molecules will move randomly through it.
2. Bioenergetics of membranes. It is important to understand how cell energy is obtained. Movement from A to B requires an input of energy. This energy can e chemical (ATP) or physical (concentration gradient). In an uncharged membrane system, there are 2 infinitely large solutions containing X at 2 concentrations [X]sta and [X]fin How much energy is required to move 1 mol of X from [X] sta to [X]fin. ΔG (kJ mol-1) ln ([X]fin/[X]sta) x T
•
If there is a negative ΔG, means something has less disorder, or more order.
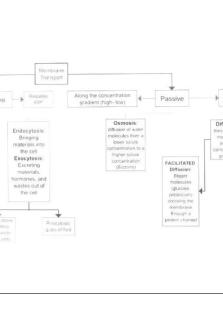
3. Types of transport mechanism. - PASSIVE o Simple diffusion o Facilitated diffusion - ACTIVE o Primary active transport (ATP dependent) o Secondary active transport (Coupled transporter) Simple diffusion For small molecules: water through osmosis, carbon dioxide and oxygen. Transport of small lipid soluble molecules: Hormones, drugs, and toxins. It is done by lipid bilayers are not permeable to ions (K+, Na+, Ca2+, Cl-, HCO3-), small hydrophilic molecules like glucose and macromolecules like proteins and RNA. Facilitated diffusion
Allows passage of charged hydrophilic or bulky molecules through membrane (e.g. glucose). The speed they move is exponentially decreasing.
4. The phospholipid bilayer It is made of phospholipids; each has a polar head group and a hydrophobic core.
It is the primary membrane surrounding cells. Some of them, like Gramm+ only has 1 phospholipid bilayer whereas the Gramm- has 2 membrane.
In prokaryotes- cytoplasmic membrane, outer membrane In eukaryotes- endoplasmic reticulum, outer membrane, and Golgi apparatus. The reason why phospholipids form bilayers is because of the structure of the individual units that make it up. -
Micelles: single FA Vesicles- double FAs
5. The plasma membrane. The cells membrane contains more things phospholipids. It is not a solid structure but a fluid-mosaic model. Membrane proteins have hydrophobic surfaces that associate with membrane. They come in different forms and shapes and they all have charged regions that give them the way of how are located in the cell.
6. Lateral diffusion bilayer How do these proteins fit into the membrane? They are held in place by the hydrophobic bands that wrap around the structure. Due to the fluid-mosaic model, the protein can move anywhere laterally, and in/out of the lipid bilayer. If the protein tries to rotate, the charges will go inside the hydrophobic part of the membrane, so it will be repulsed.
7. Transporters VS catalysts. Transporter proteins lower the energy required to traverse the membrane. Catalytic enzymes lower the energy required to change substrate to product....
Similar Free PDFs
Membrane Transport Flow Chart
- 1 Pages

Week 2 Membrane transport lab
- 6 Pages

Chapter 7 HW Membrane Transport
- 1 Pages

Introduction to Payroll - week 8
- 5 Pages

Chap 8 - Transport in Animals
- 13 Pages
Popular Institutions
- Tinajero National High School - Annex
- Politeknik Caltex Riau
- Yokohama City University
- SGT University
- University of Al-Qadisiyah
- Divine Word College of Vigan
- Techniek College Rotterdam
- Universidade de Santiago
- Universiti Teknologi MARA Cawangan Johor Kampus Pasir Gudang
- Poltekkes Kemenkes Yogyakarta
- Baguio City National High School
- Colegio san marcos
- preparatoria uno
- Centro de Bachillerato Tecnológico Industrial y de Servicios No. 107
- Dalian Maritime University
- Quang Trung Secondary School
- Colegio Tecnológico en Informática
- Corporación Regional de Educación Superior
- Grupo CEDVA
- Dar Al Uloom University
- Centro de Estudios Preuniversitarios de la Universidad Nacional de Ingeniería
- 上智大学
- Aakash International School, Nuna Majara
- San Felipe Neri Catholic School
- Kang Chiao International School - New Taipei City
- Misamis Occidental National High School
- Institución Educativa Escuela Normal Juan Ladrilleros
- Kolehiyo ng Pantukan
- Batanes State College
- Instituto Continental
- Sekolah Menengah Kejuruan Kesehatan Kaltara (Tarakan)
- Colegio de La Inmaculada Concepcion - Cebu










