Effect of sodium hydroxide on methyl benzoate PDF

| Title | Effect of sodium hydroxide on methyl benzoate |
|---|---|
| Course | Chemistry |
| Institution | Trinity College Dublin University of Dublin |
| Pages | 5 |
| File Size | 226.1 KB |
| File Type | |
| Total Downloads | 115 |
| Total Views | 171 |
Summary
#2 Research into the effect of Sodium hydroxide on methyl benzoate Objectives: To investigate the effects that Sodium hydroxide has on Methyl benzoate. To determine whether or not the reaction produces an acidic or a basic product. To identify the product of the reaction between Sodium h...
Description
#2 Research into the effect of Sodium hydroxide on methyl benzoate Objectives:
To investigate the effects that Sodium hydroxide has on Methyl benzoate. To determine whether or not the reaction produces an acidic or a basic product. To identify the product of the reaction between Sodium hydroxide and Methyl benzoate. To further develop confidence with the use of Reflux apparatus. To practise Recrystallization as a purification technique. To determine the melting point of a product to determine the purity and help in the identification of the product.
Theory: When Methyl benzoate is shaken with water two liquid layers form. The upper layer is Methyl benzoate (less dense) and the lower layer is water. There is no clear indication of any reaction taking place. A more careful study shows that Methyl benzoate reacts very slowly with water and is hydrolysed to give benzoic acid and Methanol but the reaction does not go to completion. However, Methyl benzoate is found to react much faster with aqueous sodium hydroxide, the reaction going to completion, to give sodium benzoate (water soluble) and Methanol (miscible with water). This process is called base hydrolysis (or saponification) of an ester and is used in this experiment to first obtain sodium benzoate solution, and then benzoic acid from Methyl benzoate. The Methanol may be recovered by simple downward distillation from the reaction mixture and collected as a solution in water. But this step is omitted in this experiment to allow it to be completed in the available time. The sodium benzoate is non-volatile and remains in solution. Treatment of this solution with hydrochloric acid releases the free benzoic acid allowing the Crystals to form. There are two advantages to using an alkali as opposed to an acid in Hydrolysis. The reactions are one-way rather than being reversible and also the products are easier to separate. Hydrolysis is the chemical breakdown of a compound due to the presence of water. In the case of this experiment the acid was wanted as opposed to the salt sodium benzoate so excess Hydrochloric acid is added to the solution after the first dilution. Hence the solution is flooded with Hydrogen Ions. The ions are picked up by the benzoate ions to produce Benzoic Acid. Recrystallization is a process used to eliminate any impurities in a Chemical, in order to have it in its purest form. In theory the melting point of Benzoic acid is 122.41 degrees Celsius.
Experimental Procedure: Set up apparatus for Reflux. Weigh out 2g of Methyl benzoate and measure 45mL of 1M Sodium hydroxide in a 100mL round bottomed flask add 5 boiling chips. Record any observations upon addition of the two substances. Heat the Solution under Reflux for 30 minutes. Observe and Record what happens when the mixture appears to have gone to completion, cool the mixture to room temperature. Pour the mixture into a beaker and add Concentrated Hydrochloric acid (5cm^3) drop wise. Place the Beaker in an Ice bath to cool the solution and to aid the formation of Crystals. Filter the Crystals with the use of Buchner Filtration. Weigh the Crude product of the Crystals in a weigh boat. Place approx. 0.12g of the Crystals in a solution of 10cm^3 DCM in a separate beaker and add Litmus paper.
Observe the change in colour of the Litmus Paper. Take a sample of the Crude Crystals for Recrystallization. Hence dissolve the Crystals in 10mL Deionised water. Stir with a glass rod to dissolve the Crystals. Heat on a hot plate until all of the Crystals have dissolved. Take a sample of the purified Crystals and test with Sodium bicarbonate. Record any observations. Take a TLC plate of the Crude Crystals and Purified Crystals. Draw a line in pencil 1cm above the end of the plate. Draw 4 dots and label them (SM-starting material C-control Iimpure-pure).Place a spot of Methyl benzoate, using a Capillary tube on SM, hence a spot of Methyl benzoate, impure and pure Crystals on the Control-C. Spot the impure on I and pure on P. Place 10cm^3 DCM in a lidded Jar and add the TLC plate. Replace the lid and leave for 15 minutes. Hence remove the plate and mark the solvent layer with a pencil. Allow to dry and furthermore examine the plate under UV light and mark the spots by drawing pencil circles around them on the plate. Test the Melting point of the purified product and Crude product. To test the melting points place a sample of both the crude product and pure product each in a Capillary tube sealed at one end. Tap the bench with the tube until all the powder falls to the end of the tube. Before placing the tubes into the Boiling apparatus, check the temperature with a Thermometer to ensure the temperature is below 50 degrees Celsius. Place each sample into a slot and heat it slowly, watch the samples all of the time. Once a sample begins to melt, record the range of temperature over which the sample melts. Calculate the Rf values from the TLC and the percentage yield of the pure and the impure Crystals. Results: Upon the addition of the Sodium bicarbonate (alkali) to the Crude product there was a lot of fizzing and a gas was given off, indicating that the product was acidic. Also upon the addition of HCL crystals started to form. In addition, a solution of the crude product in DCM turns blue litmus paper pink, which also verifies the result that the product is acidic. The boiling point of the Crude product was 110 degrees Celsius and the range of the melting point of the pure was 110-117 degrees Celsius. Percentage yield of the Crude Product: C8H8O2= Methyl benzoate. 8(12) +8+2(16) =136g. 2g Methyl benzoate used. 1M- 136g ? - 2g 2/136= 0.01470 moles of Methyl benzoate. C7H602= Benzoic acid. 7(12) + 6 + 2(16) = 122g. 122 x 0.01470 = 1.7934g. 1.7934g is the Theoretical yield of benzoic acid. 4.01g is the Actual yield of Crude Crystals.
Percentage yield = Actual yield/ Theoretical Yield x 100. 4.01g/1.7934g x 100 = 223.59%. Percentage yield of Product after Recrystallization: 0.46g of Crude Crystals taken for Recrystallization. 1.7934g is the Theoretical yield of Benzoic acid. 0.05g of Crystals were left after Recrystallization. 0.05g/1.7934g x 100 = 2.788% Percentage yield. 0.46g-0.05g = 0.41g in impurities.
TLC Plate: Under UV light:
Retention Factor: 0.5cm/6.5cm = 0.076923cm. Rf = Distance travelled by Compound. /Distance travelled by solvent front. Calculation of Retention Factor:
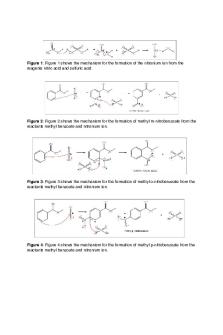
Rf = 0.5cm/6.5cm = 0.076923cm. Rf= 0.076923cm. Discussion: The first step in the reaction of Methyl benzoate with aqueous Sodium hydroxide is the attack of the nucleophile HO^- on the Carbonyl Carbon atom, forming a tetrahedral intermediate. This can either eject HO^-, to give the starting materials or eject the CH3O^anion, to give the acid:
The reaction is completed by the basic methoxide ion reacting with a proton from the Benzoic acid.
The last step in the mechanism is essentially irreversible, because methanol is a much weaker acid than Benzoic acid. This makes the base hydrolysis of the ester an irreversible process therefore, for this reason ester hydrolysis is usually carried out under basic Conditions. The various tests performed on the Crude product identified it as being Benzoic Acid. The percentage yield of the Crude acid is so high due to there being a lot of impurities within the Benzoic acid, perhaps some Methyl benzoate was still present. In addition the fact that a 0.46g sample of the crude product was taken for Recrystallization and only 0.05g of the
Crystals were hence left exhibits how high the level of impurities (0.41g)were within the sample. The boiling point of the Crude product was 110 degrees Celsius indicating once again the level of impurities within the sample as in theory the melting point is 122.41 degrees Celsius. The Range of the melting point for the purified Crystals was 110-117 degrees Celsius, indicating how there was still a degree of impurities within the sample, but one could conclude that the sample that was recrystallized is much purer than the crude sample, as it’s melting point is much closer to the theoretical one. With regards to the TLC Plate, Methyl benzoate is less polar than Benzoic acid, as it is attracted to the solvent front. Benzoic acid has a much lower retention factor than that of Methyl benzoate, as Benzoic acid is much more polar than Methyl benzoate.
Conclusion:
Recrystallization is a highly effective way of purifying a sample. Methyl benzoate and Sodium hydroxide react to produce a simple, carboxylic acid Benzoic acid. Rotary evaporation is an efficient method for the easy and relatively quick removal of Solvents from Samples by evaporation. An acid turns blue litmus paper red. The melting point of a substance attained experimentally is a good indicator of its purity, when compared to its theoretical melting point....
Similar Free PDFs

Nitration of Methyl Benzoate
- 4 Pages

Nitration of Methyl Benzoate
- 2 Pages

Nitration of Methyl Benzoate
- 9 Pages

EXP 2 - Sodium Benzoate
- 4 Pages
Popular Institutions
- Tinajero National High School - Annex
- Politeknik Caltex Riau
- Yokohama City University
- SGT University
- University of Al-Qadisiyah
- Divine Word College of Vigan
- Techniek College Rotterdam
- Universidade de Santiago
- Universiti Teknologi MARA Cawangan Johor Kampus Pasir Gudang
- Poltekkes Kemenkes Yogyakarta
- Baguio City National High School
- Colegio san marcos
- preparatoria uno
- Centro de Bachillerato Tecnológico Industrial y de Servicios No. 107
- Dalian Maritime University
- Quang Trung Secondary School
- Colegio Tecnológico en Informática
- Corporación Regional de Educación Superior
- Grupo CEDVA
- Dar Al Uloom University
- Centro de Estudios Preuniversitarios de la Universidad Nacional de Ingeniería
- 上智大学
- Aakash International School, Nuna Majara
- San Felipe Neri Catholic School
- Kang Chiao International School - New Taipei City
- Misamis Occidental National High School
- Institución Educativa Escuela Normal Juan Ladrilleros
- Kolehiyo ng Pantukan
- Batanes State College
- Instituto Continental
- Sekolah Menengah Kejuruan Kesehatan Kaltara (Tarakan)
- Colegio de La Inmaculada Concepcion - Cebu