Nitration of Methyl Benzoate PDF

| Title | Nitration of Methyl Benzoate |
|---|---|
| Course | Organic Chemistry Laboratory Ii |
| Institution | University of Miami |
| Pages | 9 |
| File Size | 600 KB |
| File Type | |
| Total Downloads | 13 |
| Total Views | 145 |
Summary
lab report...
Description
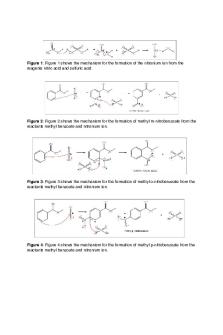
Page 1 of 9 Nitration of Methyl Benzoate: A Macroscale Synthesis Objective: To perform an electrophilic aromatic substitution, and to run this reaction on a macroscale. In this experiment, we treat nitric acid with sulfuric acid to produce methyl mnitrobenzoate. Introduction: A hydrogen on the aromatic ring is replaced by an electrophilic reagent in an electrophilic aromatic substitution. Aromatic compounds are surrounded by π-electron clouds. This makes them very unreactive to nucleophiles. However, we need highly reactive electrophilic reagents because a reaction to aromatic ring results in loss of resonance stabilization. For example, in this experiment nitronium ion is a highly reactive electrophilic reagent. It is formed with a mixture of nitric acid and concentrated sulfuric acid. Nitric acid acts as base. We use methyl benzoate as the substrate in this experiment because methyl ester group is a deactivating group which will make the experiment safer.
Safety hazards: Nitric acid and sulfuric acid are both very strong acids, and are corrosive. Avoid acid spills on the skin. Procedure and Observations: Procedure Temperature of the experiment: below 15 degrees celsius. Place 12 mL of concentrated sulfuric acid in a 100mL beaker. Cool to about 0°C using an ice-water bath. Add about 6 g of methyl benzoate using a graduated cylinder Cool it to 0°C.
Observations Avoids excessive formation of byproduct.
Page 2 of 9 Procedure Add a cooled to 0°C mixture of 4 mL of nitric acid and 4mL of sulfuric acid using a Pasteur pipet.
Observations This was done slowly.
Stir with a glass rod. Keep the temperature below 15 degrees celsius using an ice bath.
The temperature controls how fast we can add the sulfuric nitric acid mixture.
Allow the mixture to warm to room temperature. Wait for 15 minutes. Pour this reaction mixture onto 50 g of ice in a larger beaker. Filter the mixture when the ice has melted. Wash the solids on the filter twice with 25 mL of cold water, and twice with 10 mL of cold methanol. Weigh the crude prod- uct and recrystallize from methanol. Filter and dry the crystals on top of the Büchner funnel.
Used very minimum amount of methanol.
Page 3 of 9
Data Collection/ Calculations: Density of Methyl benzoate: 1.084 g/mL Volume of Methyl Benzoate: 5.54 mL Crude product: 7.224 g Literature melting point: 78 degrees celsius Experimental melting point: 78.9 degrees celsius Recrystallized product: 5.031 g Percent yield of crude product: 7.224/7.86g x 100% = 91.90% IR Spectrum:
Page 4 of 9 NMR:
Page 5 of 9
Page 6 of 9
Discussion: We performed an electrophilic aromatic substitution. We treated nitric acid with sulfuric acid to produce methyl-m-nitrobenzoate. We obtained the methyl m-benzoate product, we saw a peak at 3092.22 cm-1. This means there’s C-H bonds. The peak at 1715 cm-1 indicates a carbonyl group while the peak at 1526 cm-1 indicates a C-NO2 group. We recovered majority of the product with the yield of 91.90%. The IR spectrum matches the one in lab manual. Electrophilic aromatic substitution is an organic reaction in which an atom is attached to an aromatic system and is replaced by an electrophile. In this particular experiment, methyl benzoate was reacted with nitric acid to form methyl-m-nitrobenzoate. We performed this experiment on a macroscale. Some advantages to the microscale reactions relative to macroscale reactions include increased safety, sturdier glassware, and less chemical waste. The methyl ester group of the methyl benzoate is a meta director so the main product of the reaction is methyl mnitrobenzoate. Tthe temperature of the reaction has to be kept at or below 15 degrees C. The literature value for the melting point was 78 degrees celsius and our experimental melting point was 78.9 degrees celsius. This is close to the literature value indicating the reaction was successful. The percentage yield calculated was 91.90%. Source of error that could have caused impurities could be if the temperature was not kept below 15 degrees celsius. If the crude product was left behind during retrieval from the beaker after washing, that could have been a reason for a lower percent yield. The temperature is not controlled it would produce excessive formation of byproducts, including a dinitrated product or otho/para- substituted compounds. Overall, the experiment was successful and we learnt how to perform an electrophilic aromatic substitution in a macroscale reaction.
References: Department of Chemistry. (2015-2017). CHM 205/206 Lab Manual. Miami, Florida: University of Miami at Coral Gables. Padias, Anne B “Making the connections: A how to guide for organic chemistry lab techniques”. New York: Hayden McNeil, 2007.
Page 7 of 9 Post lab questions: 1.
Page 8 of 9
2. Sulfuric acid can make it ionic by protonating it. It will then be highly polar and be ready to dissolve. 3.
4. Methyl m-Nitrobenzoate is formed in this reaction rather that ortho/para isomers because of the ester group of the starting product of methylbenzoate. The functional group of ester is an electron withdrawing group causing nitrobenzene (NO2) to be in the meta position. Hence NO2 is a deactivating group causing itself to be a meta director.
Page 9 of 9 5.
6. The interpretation of the IR spectrum of methyl mNitrobenzoate: 1710 cm-1 correspond to C=O stretch of ester! 750 cm-1 for meta distribution of benzene! 1500-1600 cm1 peaks for CC aromatic stretch 3000-3100 cm1 aromatic =CH stretch! 1550-1475 cm-1 and 1360-1290 cm-1 for NO stretch when attached to aromatic ring. 7. To remove any side product and unreacted reagents. During the wash, the product is less soluble in cold methanol. So no product dissolves in methanol. 8. So that the compound does not remain in the liquid after cooling and all the compound comes out as crystals from the solvent. 9. HSO410. D 11. C 12. A...
Similar Free PDFs

Nitration of Methyl Benzoate
- 4 Pages

Nitration of Methyl Benzoate
- 2 Pages

Nitration of Methyl Benzoate
- 9 Pages

preparation of benzyl benzoate
- 6 Pages
Popular Institutions
- Tinajero National High School - Annex
- Politeknik Caltex Riau
- Yokohama City University
- SGT University
- University of Al-Qadisiyah
- Divine Word College of Vigan
- Techniek College Rotterdam
- Universidade de Santiago
- Universiti Teknologi MARA Cawangan Johor Kampus Pasir Gudang
- Poltekkes Kemenkes Yogyakarta
- Baguio City National High School
- Colegio san marcos
- preparatoria uno
- Centro de Bachillerato Tecnológico Industrial y de Servicios No. 107
- Dalian Maritime University
- Quang Trung Secondary School
- Colegio Tecnológico en Informática
- Corporación Regional de Educación Superior
- Grupo CEDVA
- Dar Al Uloom University
- Centro de Estudios Preuniversitarios de la Universidad Nacional de Ingeniería
- 上智大学
- Aakash International School, Nuna Majara
- San Felipe Neri Catholic School
- Kang Chiao International School - New Taipei City
- Misamis Occidental National High School
- Institución Educativa Escuela Normal Juan Ladrilleros
- Kolehiyo ng Pantukan
- Batanes State College
- Instituto Continental
- Sekolah Menengah Kejuruan Kesehatan Kaltara (Tarakan)
- Colegio de La Inmaculada Concepcion - Cebu