Esterification of benzoic acid to give methyl benzoate PDF

| Title | Esterification of benzoic acid to give methyl benzoate |
|---|---|
| Author | Sarah Campian |
| Course | Inorganic chemistry |
| Institution | Trinity College Dublin University of Dublin |
| Pages | 7 |
| File Size | 183.1 KB |
| File Type | |
| Total Downloads | 34 |
| Total Views | 135 |
Summary
benzoic acid to give methyl benzoate...
Description
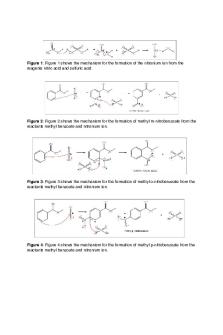
Experiment 1: Esterification of Benzoic Acid to give Methyl Benzoate Objectives: The objectives of this experiment is to heat a reaction mixture under reflux and to investigate the use of a separating funnel to allow for solvent extraction, to examine the use of a rotary evaporator and thin layer chromatography and to understand how benzoic acid undergoes esterification in methanol to produce the ester methyl benzoate. Theory: Fig 1.1 : Esterification mechanism of benzoic acid with methanol
Fischer esterification is the conversion of carboxylic acids to esters using acid catalysts and alcohols. In this experiment benzoic acid reacted with methanol (alcohol) in the presence of concentrated sulfuric acid (catalyst) to allow for the formation of methyl benzoate and water. In the esterification mechanism above (Fig 1.1) the addition of a proton leads to a more reactive electrophile. The nucleophilic attack of the methanol gives two hydroxyl groups. One is eliminated after tautomerism (proton shift) to give water and the ester, methyl benzoate. This reaction occurs at equilibrium. The sulfuric acid speeds up the reaction by lowering the activation energy to allow for a faster process. The reaction mixture is boiled in order to maintain a constant temperature for this reaction, in doing so some of the reaction solvent vaporizes, therefore it is necessary to arrange for the vapor to condense and return to its original liquid state. This is accomplished by heating under reflux and attaching a condenser to the top of the flask containing the mixture. This allows the esterification process to occur at a higher temperature thereby increasing the rate of reaction. The ester synthesized is impure and contains inorganic substances (sulfuric acid and water) and organic substances (ester, carboxylic acid and methanol). The ester must be separated from the inorganic substance and this process is achieved using a separating funnel. The separating funnel isolates the ester since all the other substances apart from the ester itself is miscible with water. The ester has a low solubility and the aqueous solution forms a layer on top of the organic solution. After washing, traces of acid are still present in the ester layer therefore 5% sodium carbonate is added to neutralize the remaining acid. The addition of sodium carbonate
forms bubbles of carbon dioxide but the bubbles stop forming once the acid is completely neutralized. Following the separation of the organic layer there may still be traces of water left behind. The solution to this is to add anhydrous magnesium sulfate which acts as a drying agent. An involatile organic material in a volatile solvent remains after the solvent extraction. The solvent is removed using a rotary evaporator. This functions by reducing the pressure to lower the boiling point of the solvent. The sample is also rotated to encourage evaporation and to increase the effective surface area. Thin Layer Chromatography (TLC) is a chromatographic technique used to separate the components of a mixture using a thin stationary phase by an inert backing. In this experiment the stationary phase is silica on a sheet of aluminum foil. The separation principle of TLC states that the separation relies on the relative affinity of compounds towards the stationary phase and the mobile phase. The mobile phase is a development jar that contains a little of the developing solvent. This method monitors reactions by analyzing the disappearance of products of starting materials and the appearance of products as a reaction proceeds. TLC compares the Retention Factor (Rf) of a known compound to that of an unknown compound. Rf is defined as the distance travelled by the compound divided by the distance travelled by the solvent front. Experimental Procedure: Benzoic acid (10.012 g) was dissolved in methanol (40 mL) in a beaker. This solution was poured into a round bottomed flask (100 mL). A sample for thin layer chromatography (TLC) was taken using a capillary tube. Concentrated sulfuric acid (2 mL) was carefully added into the round bottomed flask and the flask was swirled to ensure the mixture was homogenous. Several boiling chops were added to the round bottomed flask. The apparatus was set up for heating under reflux and the solvent was removed under reduced pressure. The solution was heated in a heating mantle for 30 minutes. The reaction mixture was cooled for 10 minutes and was poured into water (100 mL) in a separating funnel with the tap closed. Two additions of dichloromethane (2x40 mL) were added. A stopper was placed in the funnel and held in place while the funnel was inverted so that the outlet was directed upwards. The tap was opened with the funnel inverted to release pressure build up inside and it was closed once again. This step was repeated several more times until two distinct layers were visible. The organic layer (the lower layer) was drained into a conical flask. The aqueous layer (the upper layer) was poured into a beaker. The organic layer (DCM) was poured into the separating funnel. The same procedure as before was followed but dichloromethane and sodium carbonate (5% ,30 mL) was used to remove unreacted benzoic acid. The upper aqueous layer was discarded, and the dichloromethane layer was washed with water. The lower organic layer was separated, and it was dried using anhydrous magnesium sulfate (drying agent). The drying agent was filtered off. The dichloromethane solvent was evaporated from my product using the rotary evaporator. A clean, dry, empty beaker was
weighed, and the methyl benzoate collected was placed into this beaker. The beaker was weighed once again containing the product. The percentage yield was calculated. A sample was taken using a capillary tube for TLC and a TLC plate of the starting materials and products was run. The retention factor was calculated from the results of the TLC.
RESULTS AND DISCUSSION Results: Weight of: Benzoic acid Empty round bottom flask Round bottom flask & distilled methyl benzoate Methyl benzoate
Grams (g) 10.012 47.720 52.749 5.029
Benzoic acid= C7H6O2 1 mole of benzoic acid = 7(12) +6(1) +2(16) = 122 g 10.012 g =
10.012 122
= 0.0821 moles
Methyl benzoate= C8H8O2 1 mole of methyl benzoate= 8(12) + 8(1) + 2(16) = 136 g Theoretical yield of methyl benzoate= 136 × 0.0821 = 11.1656 g Percentage yield=
Actual yield Theoretical yield
× 100 =
5.029 g 11.1656 g
× 100 = 43.15%
Thin Layer Chromatography: Fig 1.2: TLC plate viewed under ultraviolet light
Solvent front = 9.3 cm Methyl benzoate = 8.4 cm
A = 4.5 cm
B = 1.8 cm
Retention factor (Rf) =
Rf of compound =
Distance travelled by compound Distance travelled by solvent front
8.4 cm 9.3 cm
= 0.903
Rf of component (A) = 0.484 Rf of component (B) = 0.194 Discussion: The importance of calculating a theoretical yield and an actual yield is to account for the accuracy and success of an experiment. The actual yield of the methyl benzoate (5.029 g) was very different to the theoretical yield of the methyl benzoate (11.163 g). The percentage yield was only 45%. There was a possible source of error during the process of inverting the funnel. Whilst doing this I waited too long to open the tap to release the pressure in the funnel. This resulted in the solution spilling out of the separating funnel due to having a buildup of pressure, causing us to lose most of the methyl benzoate in solution. Therefore, this experiment did not work out as I predicted. There may have also been other possible sources of error when weighing the round bottomed flask, to result in a quantitative error. There may have been miscalculations when calculating the number of moles of benzoic acid produced caused by incorrect rounding. Impure samples and unclean and dusty equipment may have also contributed to the results of this experiment. The Rf of component A (benzoic acid) turned out to be greater than that of component B (methyl benzoate). The more polar a substance is, the longer the distance it travels in TLC. The carboxylic group of benzoic acid is polar although the molecule overall is nonpolar. Methyl benzoate is a non-polar compound. Due to the polarity of the carboxylic group, the benzoic acid travelled a longer distance (4.5 cm) than the methyl benzoate (1.8 cm). The purpose of calculating the Rf of each component is to provide a comparison against which other compounds can be judged. The Rf calculation also accounts for the purity of the final product and whether all the benzoic acid was removed or not. Some of the benzoic acid remained on the TLC plate which may have been caused by not shaking the dichloromethane solution and sodium carbonate until homogenous. Conclusion: The esterification of benzoic acid with methanol requires a catalyst, sulfuric acid to produce an ester, methyl benzoate and water. For this reaction to occur the reaction needs heat which is provided when the benzoic acid, methanol and sulfuric acid is under reflux and a catalyst to speed up the reaction without itself being used up un the process. In order to obtain a pure sample of methyl benzoate the ester must be separated using various techniques including the use of a separating funnel and a rotary evaporator. Thin layer chromatography measures the purity of the sample of the methyl benzoate.
Post practical questions: 1. Acid- catalyzed esterification of pentanoic acid with ethanol
2. Esters can be produced by reacting acyl chlorides with alcohol. For example, the reaction between ethanoyl chloride and ethanol forming ethyl ethanoate.
3. The mixture is treated with aqueous sodium hydrogen carbonate to remove any unreactive benzoic acid to obtain a purer product and to allow for a more accurate result.
2 C6H5COOH + Na2CO3
2 C6H5COONa + CO2 + H2O...
Similar Free PDFs

Nitration of Methyl Benzoate
- 4 Pages

Nitration of Methyl Benzoate
- 2 Pages

Nitration of Methyl Benzoate
- 9 Pages

Benzoic Acid
- 9 Pages
Popular Institutions
- Tinajero National High School - Annex
- Politeknik Caltex Riau
- Yokohama City University
- SGT University
- University of Al-Qadisiyah
- Divine Word College of Vigan
- Techniek College Rotterdam
- Universidade de Santiago
- Universiti Teknologi MARA Cawangan Johor Kampus Pasir Gudang
- Poltekkes Kemenkes Yogyakarta
- Baguio City National High School
- Colegio san marcos
- preparatoria uno
- Centro de Bachillerato Tecnológico Industrial y de Servicios No. 107
- Dalian Maritime University
- Quang Trung Secondary School
- Colegio Tecnológico en Informática
- Corporación Regional de Educación Superior
- Grupo CEDVA
- Dar Al Uloom University
- Centro de Estudios Preuniversitarios de la Universidad Nacional de Ingeniería
- 上智大学
- Aakash International School, Nuna Majara
- San Felipe Neri Catholic School
- Kang Chiao International School - New Taipei City
- Misamis Occidental National High School
- Institución Educativa Escuela Normal Juan Ladrilleros
- Kolehiyo ng Pantukan
- Batanes State College
- Instituto Continental
- Sekolah Menengah Kejuruan Kesehatan Kaltara (Tarakan)
- Colegio de La Inmaculada Concepcion - Cebu