Nitration of Methyl Benzoate Discussion PDF

| Title | Nitration of Methyl Benzoate Discussion |
|---|---|
| Course | Organic Chemistry Laboratory II |
| Institution | University of Arizona |
| Pages | 2 |
| File Size | 79.2 KB |
| File Type | |
| Total Downloads | 42 |
| Total Views | 135 |
Summary
The TA's name was Kayla Bates....
Description
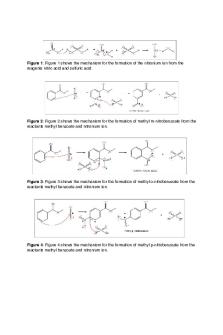
Laboratory 3B: Nitration of Methyl Benzoate, a Macroscale Synthesis Don’t forget to turn in your NMR spectrum with complete analysis. Short response 1. Define “electrophile” and explain what makes the nitronium ion an electrophile. An electrophile is a reagent that wants electrons. They generally have a positive charge on one of the atoms or they have a slight positive charge due to other atoms in the molecule with a higher electron density. A nitronium ion is positively charged, so it wants electrons in order to get back to a neutral, and therefore more stable state. It will be highly reactive with a nucleophile, in this case the methyl benzoate.
2. While the meta-substituted product is the major one formed in this reaction, smaller amounts of both the ortho and para products are also produced. In which part of the procedure do you expect those by-products to be removed? The ortho and para products were formed with the meta product as the two acids were added to the methyl benzoate. After obtaining the crude product, we used recrystallization as a means to purify the meta product. After the recrystallization procedure is complete, I would expect the ortho and para products to be removed. Pure meta-substituted methyl nitrobenzoate should be all that is left over.
3. Did your NMR spectrum of your compound indicate that it was pure? Explain. The NMR spectrum of the product did show traces of impurities (leftover methanol, chloroform, etc), but overall it was a pure sample. The only issue I had with assigning peaks was that there are five different hydrogen types in methyl m-nitrobenzoate, but my NMR only had four major peaks. The only thing I can think of was that two of the hydrogen types that are located in the benzene ring were similar enough to be grouped as one when the NMR was run. If this is what happened, then my sample was pure with only trace amounts of impurities (those impurities can also be confirmed by looking at the melting point).
4. Why is it necessary to maintain the reaction temp below 15 °C? The reaction needed to be kept under 15°C because if it goes too far over for too long, more byproducts will be formed (ortho and para products). If more of those products are formed, the yield will be less because more of those products have to be removed, leaving you with a smaller amount of the meta product. This could also cause more impurities in the sample if the recrystallization does not get rid of all the byproducts. This would show up in the melting point value and the NMR spectra.
Nitration of Methyl Benzoate
Dana Woods CHEM 243B Section 43 TA: Kayla...
Similar Free PDFs

Nitration of Methyl Benzoate
- 4 Pages

Nitration of Methyl Benzoate
- 2 Pages

Nitration of Methyl Benzoate
- 9 Pages

preparation of benzyl benzoate
- 6 Pages
Popular Institutions
- Tinajero National High School - Annex
- Politeknik Caltex Riau
- Yokohama City University
- SGT University
- University of Al-Qadisiyah
- Divine Word College of Vigan
- Techniek College Rotterdam
- Universidade de Santiago
- Universiti Teknologi MARA Cawangan Johor Kampus Pasir Gudang
- Poltekkes Kemenkes Yogyakarta
- Baguio City National High School
- Colegio san marcos
- preparatoria uno
- Centro de Bachillerato Tecnológico Industrial y de Servicios No. 107
- Dalian Maritime University
- Quang Trung Secondary School
- Colegio Tecnológico en Informática
- Corporación Regional de Educación Superior
- Grupo CEDVA
- Dar Al Uloom University
- Centro de Estudios Preuniversitarios de la Universidad Nacional de Ingeniería
- 上智大学
- Aakash International School, Nuna Majara
- San Felipe Neri Catholic School
- Kang Chiao International School - New Taipei City
- Misamis Occidental National High School
- Institución Educativa Escuela Normal Juan Ladrilleros
- Kolehiyo ng Pantukan
- Batanes State College
- Instituto Continental
- Sekolah Menengah Kejuruan Kesehatan Kaltara (Tarakan)
- Colegio de La Inmaculada Concepcion - Cebu