Nitration of Methyl Benzoate PDF

| Title | Nitration of Methyl Benzoate |
|---|---|
| Author | April |
| Course | Organic Chemistry II |
| Institution | Rhode Island College |
| Pages | 4 |
| File Size | 102.3 KB |
| File Type | |
| Total Downloads | 121 |
| Total Views | 159 |
Summary
Nitration lab...
Description
Nitration of Methyl Benzoate Alyson April Savattere, PBT (ASCP) CPC (AAPC) Organic Chemistry 206, Rhode Island College, Providence, Rhode Island 02908 [email protected] February 26, 2019
Abstract The nitration of methyl benzoate is an electrophilic aromatic substitution reaction that produces a substituted derivative of benzene, methyl-3-nitrobenzoate, as the major product. The synthesis of methyl-3nitrobenzoate proceeded by way of an electrophilic nitronium ion reacting with a protonated intermediate to create a cyclohexadienyl cation intermediate. The reaction is complete when the aromaticity of the ring is restored by the loss of a proton (H+) from the cyclohexadienyl cation to give methyl-3-nitrobenzoate (4).
spectrometer. Data analysis of the NMR spectra was completed using NUTS software by Acorn, Inc. Quantitation of the purity of the final product is given by the GC-MS (Varian Saturn 2200 GC-MS with a Varian CP3800 GC; VF-5ms 30 m x 0.25 mm I.D. (DF = 0.25) column) spectrum. Analysis of the total energy (kcal/mol) and heats of formation (kcal/mol) of methyl benzoate, para-nitro and the meta-nitro reaction intermediates were completed by utilizing the computational chemistry software program, Semi-empirical Geometry Optimization using PM3 with HyperChem 8.0.
Experimental Section General Synthetic Methods Due to the formation of a water-sensitive nitronium ion during the reaction, the glassware being used were dried thoroughly prior to the start of the experiment (2). Several tests were performed to analyze the substitution pattern and level of purity of the crude and pure products. Infrared spectroscopy is performed using a Perkin Elmer Spectrum 100 FTIR. The IR spectrum data was used to ascertain the presence (or absence) and substitution pattern of the nitro group. NMR spectra were recorded using the NMR Hitachi R-1200 RS NMR
Experimental Methods A dry, empty, reaction tube was placed inside of a glass beaker (100mL). The reaction tube was put on a balance and tared to zero. A clean Pasteur pipet was used to obtain a small quantity of liquid methyl benzoate. The methyl benzoate is then added to the tared reaction tube dropwise until a mass of 0.293g was reached on the balance. Using a separate and clean Pasteur pipet attached to a syringe, sulfuric acid (0.6 mL) was added to a dry reaction tube. Methyl benzoate (0.27 mL or 0.30g) was then added dropwise to
the reaction tube containing the sulfuric acid (0.6 mL). The two reactants were mixed by gently flicking the tube. Two dry Pasteur pipettes, each attached to a separate syringe, were used to obtain concentrated sulfuric acid (0.2 mL) and concentrated nitric acid (0.2 mL). Next, the tube containing the reaction mixture was placed in an ice bath. Concentrated sulfuric acid (0.2 mL) and the concentrated nitric acid (0.2 mL) were added dropwise to the reaction mixture. During the addition of the acids, the mixture was gently stirred with a glass stirring rod. Temperature of the mixture was maintained so that it did not exceed 15°C. After the addition of both acids, the tube was taken off the ice bath and warmed to room temperature (23°C) for 15 minutes.
purify the crude product, an equal mass of methanol (0.2 mL) was added dropwise. The purified product was cooled slowly for 30 minutes to complete the recrystallization. Once the recrystallization was complete, the crystals were dumped on to a piece of circular filter paper for further drying. The final product was then weighed on the balance. Finally, the purified crystals (0.054g) were used for melting point determination, GC-MS, IR, and NMR analysis. Results Melting point of the pure product was 74.878.1°C. The literature value of the meta substituted isomer has a range of 76-80°C, the para isomer is between 94-96°C, and the ortho isomer is recorded at -13°C. The melting point of our final product is in correlation with the literature value of the meta isomer. The pure product had a 13.53 % yield and the crude product had a 30.33 % yield.
Roughly 2.5g of ice was placed in a weigh boat and then added to a glass beaker (100 mL). The reaction mixture was then poured over the ice to form a solid product. The solid product formed was then collected by suction filtration using a Hirsch funnel and a flask (25 mL). The crystals were washed thoroughly with distilled water (2.0 mL) and then washed with ice-cold methanol (0.2 mL). The crude product was then removed from the top of the Hirsch funnel. Roughly 10mg of crude product was extracted for melting-point determination, percent yield, and GC-MS analysis. The mass of the remainder of the crude product was determined by using the balance.
IR analysis of the pure product (pp. 5 laboratory notebook) showed peaks at 3092, 2961, 1615, 1526, 823, 779, 1715 and 1193 cm-1 . These peaks indicate sp2 C-H stretching, sp3 C-H stretching, C=C aromatic, NO2 substitution, para and meta substitution, a C=O carbonyl group and a CC=O-C methyl ester group, respectively. The peaks at 1526 and 1349 cm-1 are long and sharp. These two peaks indicate an NO2 stretch that is symmetric and asymmetric (2).
Recrystallization of the crude product (0.121g), began by placing it in a dry glass reaction tube containing a boiling stick. To
The stereochemical assignment of the meta isomer was confirmed on the 1H NMR spectra of the pure product. Five types of
protons are indicated by the chemical shifts and splitting patterns (Table 1). Table 1. Functional Group
Proton
Splitting Pattern
Ether (H-C-OR) Aryl (Ar-H)
Ha
Singlet
Chemical Shift (ppm) 4.0
Hb
Singlet
9.0
Hc Hd He
Doublet Triplet Doublet
8.5 7.3 7.7
Table 1. describes the integrals observed on the NMR spectrum found on pp. 10 of the lab notebook.
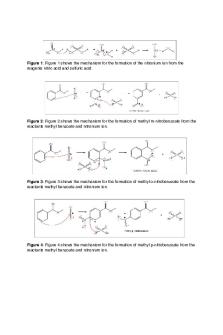
GC-MS performed on the pure product gave a chromatogram plot with four significant peaks (pp. 8-9 in laboratory notebook). The first peak has a retention time (unit = minutes) of 3.178, the second peaks retention time of 3.763 and the third peaks retention time is at 3.998. The % area of the three peaks is 0.511, 0.007, and 99.460, respectively, totaling out to 99.978%. The GC-MS software identified all three peaks as ‘Benzoic acid, 2-nitro-, meth’. The ‘2-nitro’ description indicates a nitro group that is in the ortho position on the ring. The fourth peak ‘Benzoic acid, 3-nitro, meth’ has a retention time of 4.097 and a % area of 0.021. Position 3 on the benzene ring is indicative of the meta-nitro substitution pattern. Quantitatively, the GC-MS indicates that the pure samples major isomer is ortho substituted (methyl-2nitrobenzoate) and the minor to be meta substituted (methyl-3-nitrobenzoate).
To predict the major product of the nitration of methyl benzoate, a computational portion of the lab was completed by performing semi-empirical calculations of the charge distribution in methyl benzoate and the energies of the meta-nitro and para-nitro intermediate ions. The heat of formation for methyl benzoate is -58.149 (kcal/mol). The heat of formation for the meta-nitro intermediate was found to be 152.093 (kcal/mol) and the para-nitro intermediate was 153.467 (kcal/mol). The melting point, IR, and NMR spectra of the purified product imply that the major product was methyl-3-nitro benzoate (metanitro substituted isomer). GC-MS results conflict with this implication because the spectra was from another students experiment while all other data was collected from my partners lab notebook. Therefore, we can exclude the GC-MS results when determining the effectiveness of the purification. The methods used to purify the product were highly effective and resulted in a compound with a melting point range consistent only with a metanitro substituted nitration product. Methyl3-nitrobenzoate was prepared and purified accurately and in accordance with the outlined procedure (2).
***For further analysis of the computational chem. species refer to pp. 12 of the laboratory notebook as well as the attached document, ‘Analysis of Computational Chemistry’....
Similar Free PDFs

Nitration of Methyl Benzoate
- 4 Pages

Nitration of Methyl Benzoate
- 2 Pages

Nitration of Methyl Benzoate
- 9 Pages

preparation of benzyl benzoate
- 6 Pages
Popular Institutions
- Tinajero National High School - Annex
- Politeknik Caltex Riau
- Yokohama City University
- SGT University
- University of Al-Qadisiyah
- Divine Word College of Vigan
- Techniek College Rotterdam
- Universidade de Santiago
- Universiti Teknologi MARA Cawangan Johor Kampus Pasir Gudang
- Poltekkes Kemenkes Yogyakarta
- Baguio City National High School
- Colegio san marcos
- preparatoria uno
- Centro de Bachillerato Tecnológico Industrial y de Servicios No. 107
- Dalian Maritime University
- Quang Trung Secondary School
- Colegio Tecnológico en Informática
- Corporación Regional de Educación Superior
- Grupo CEDVA
- Dar Al Uloom University
- Centro de Estudios Preuniversitarios de la Universidad Nacional de Ingeniería
- 上智大学
- Aakash International School, Nuna Majara
- San Felipe Neri Catholic School
- Kang Chiao International School - New Taipei City
- Misamis Occidental National High School
- Institución Educativa Escuela Normal Juan Ladrilleros
- Kolehiyo ng Pantukan
- Batanes State College
- Instituto Continental
- Sekolah Menengah Kejuruan Kesehatan Kaltara (Tarakan)
- Colegio de La Inmaculada Concepcion - Cebu