Nitration of Methyl Benzoate PDF

| Title | Nitration of Methyl Benzoate |
|---|---|
| Author | Maggie Tran |
| Course | (CHEM 2125, 2225, 2425) Organic Chemistry Laboratory |
| Institution | Texas A&M University |
| Pages | 2 |
| File Size | 58.4 KB |
| File Type | |
| Total Downloads | 50 |
| Total Views | 136 |
Summary
Laboratory Report Assignment that all CHEM 238 students had to do. This is the final copy of the report submitted, I got a 38/40 on this assignment. ...
Description
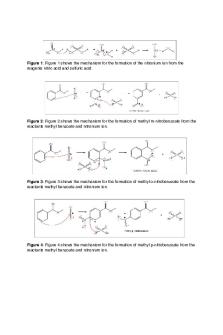
Nitration of Methyl Benzoate Results and Discussion The purpose of this experiment was to perform an aromatic substitution and from that determine the identity of the major product formed. The way this product was identified was through Thin Layer Chromatography while comparing it to other compounds. The five compounds were produced for the experiment and to use on the TLC plate to determine the Rf values in each. For the crude product and recrystallized product, the melting points were taken. The melting point range of the crude product is 59.7°-61.2°C. There were 3.797g of the crude product recovered with an 81.9% yield. The melting point range of the recrystallized product is 70°-71°C. 2.568g of the recrystallized product was recovered with a 55.4% yield. The TLC plate results were recorded and are shown in the table below.
Materials
Methyl Benzoate
Crude Product
Mother Liquor
Recrystallized product
Isomeric Mixture
Rf Values
0.512
0.341 0.280 0.207
0.341 0.293 0.207
0.317 0.232
0.390 0.341 0.244
The Rf values were eluted and are all relative the distance allowed to travel on the TLC plates and the methyl benzoate acts as a standard. For the other four compounds tested, the top Rf value represents methyl p-nitrobenzoate. The middle Rf value represents methyl mnitrobenzoate. The lower Rf value represents methyl o-nitrobenzoate. The dinitration product was the mother liquor. It can be seen from looking at the TLC plate that the crude product had a strong concentration of methyl m-benzoate. The mother liquor had strong concentrations in methyl m-benzoate and methyl o-benzoate. The recrystallized product had a very strong concentration of methyl m-benzoate. Overall this experiment was very successful because it can be determined from the results above that the major product in the final product was methyl m-benzoate. This makes sense because the substituents on the benzene ring are electron withdrawing groups which favor meta products. It can also be seen by viewing the resonance structures that the intermediates of a meta product are much more stable than the para or ortho product. This is the correct major product
that was predicted to be the outcome and shows that this method is effective at observing the electrophilic aromatic substitution.
The possible sources of error could have come from making each product and if anything was added incorrectly it could have skewed the results. If the methyl benzoate was added too quickly or not cooled down enough it could have altered the results. Also if the TLC spotting or placing in the jar was done incorrectly, the results would be extremely skewed....
Similar Free PDFs

Nitration of Methyl Benzoate
- 4 Pages

Nitration of Methyl Benzoate
- 2 Pages

Nitration of Methyl Benzoate
- 9 Pages

preparation of benzyl benzoate
- 6 Pages
Popular Institutions
- Tinajero National High School - Annex
- Politeknik Caltex Riau
- Yokohama City University
- SGT University
- University of Al-Qadisiyah
- Divine Word College of Vigan
- Techniek College Rotterdam
- Universidade de Santiago
- Universiti Teknologi MARA Cawangan Johor Kampus Pasir Gudang
- Poltekkes Kemenkes Yogyakarta
- Baguio City National High School
- Colegio san marcos
- preparatoria uno
- Centro de Bachillerato Tecnológico Industrial y de Servicios No. 107
- Dalian Maritime University
- Quang Trung Secondary School
- Colegio Tecnológico en Informática
- Corporación Regional de Educación Superior
- Grupo CEDVA
- Dar Al Uloom University
- Centro de Estudios Preuniversitarios de la Universidad Nacional de Ingeniería
- 上智大学
- Aakash International School, Nuna Majara
- San Felipe Neri Catholic School
- Kang Chiao International School - New Taipei City
- Misamis Occidental National High School
- Institución Educativa Escuela Normal Juan Ladrilleros
- Kolehiyo ng Pantukan
- Batanes State College
- Instituto Continental
- Sekolah Menengah Kejuruan Kesehatan Kaltara (Tarakan)
- Colegio de La Inmaculada Concepcion - Cebu