Green Nitration of methyl benzoate MW Lab PDF

| Title | Green Nitration of methyl benzoate MW Lab |
|---|---|
| Author | Lidya Sisay |
| Course | Organic Chemistry II |
| Institution | Collin College |
| Pages | 1 |
| File Size | 49.1 KB |
| File Type | |
| Total Downloads | 32 |
| Total Views | 149 |
Summary
notes...
Description
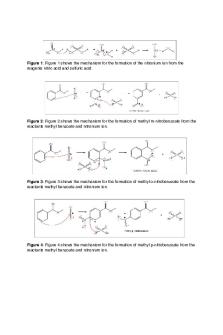
Nitration of Methyl Benzoate This reaction is of industrial importance as it is used in the synthesis of explosives, dyes, pharmaceuticals plastics and chemicals for agriculture. The reaction proceeds through the nitronium ion intermediate. This reactive particle is generated via the reaction of Cu(NO) 2 with glacial acetic acid to form HNO3 . The HNO3 then reacts as shown to yield the NO2 + intermediate.
Procedure: Combine 0.5 g Methyl benzoate and 2.5 ml Acetic acid in a 25. ml Microwave reactor vial. Slowly add Cu(NO)2 (1.2 mol Copper nitrate to 1 mol methylbenzoate) to the mixture. Upon addition of Cu(NO)2 , the color may change to black/reddish brown with brown fumes being generated. ( Should be done in the hood.) After all of the copper salt is added, the mixture is stirred for 5-10 minutes. The vial is then fitted with the pressure cap / the outer screw-on cap and placed in the microwave carrousel. Reaction is then placed in the CEM Mars 6 with a setting of 85% power,time =2 min. Upon cooling and removal from the MW unit; the reaction is removed from microwave carrousel, opened and transfer to a beaker of 10 grams of crushed ice. When Ice has melted vacuum filter, wash the solids with cold water twice. Weigh the crude product. Recrystallize from methanol. (lit mp 78 °C)...
Similar Free PDFs

Nitration of Methyl Benzoate
- 4 Pages

Nitration of Methyl Benzoate
- 2 Pages

Nitration of Methyl Benzoate
- 9 Pages

Lab 6 Nitration LAB
- 5 Pages
Popular Institutions
- Tinajero National High School - Annex
- Politeknik Caltex Riau
- Yokohama City University
- SGT University
- University of Al-Qadisiyah
- Divine Word College of Vigan
- Techniek College Rotterdam
- Universidade de Santiago
- Universiti Teknologi MARA Cawangan Johor Kampus Pasir Gudang
- Poltekkes Kemenkes Yogyakarta
- Baguio City National High School
- Colegio san marcos
- preparatoria uno
- Centro de Bachillerato Tecnológico Industrial y de Servicios No. 107
- Dalian Maritime University
- Quang Trung Secondary School
- Colegio Tecnológico en Informática
- Corporación Regional de Educación Superior
- Grupo CEDVA
- Dar Al Uloom University
- Centro de Estudios Preuniversitarios de la Universidad Nacional de Ingeniería
- 上智大学
- Aakash International School, Nuna Majara
- San Felipe Neri Catholic School
- Kang Chiao International School - New Taipei City
- Misamis Occidental National High School
- Institución Educativa Escuela Normal Juan Ladrilleros
- Kolehiyo ng Pantukan
- Batanes State College
- Instituto Continental
- Sekolah Menengah Kejuruan Kesehatan Kaltara (Tarakan)
- Colegio de La Inmaculada Concepcion - Cebu