Nitration of Methyl Benzoate Data and Mechanisms PDF

| Title | Nitration of Methyl Benzoate Data and Mechanisms |
|---|---|
| Course | Organic Chemistry II Lab |
| Institution | University of Alabama at Birmingham |
| Pages | 3 |
| File Size | 238.6 KB |
| File Type | |
| Total Downloads | 99 |
| Total Views | 154 |
Summary
Nitration of Methyl Benzoate Data and Mechanisms...
Description
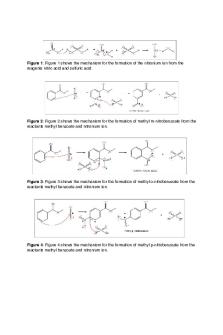
Figure 1: Figure 1 shows the mechanism for the formation of the nitronium ion from the reagents nitric acid and sulfuric acid.
! Figure 2: Figure 2 shows the mechanism for the formation of methyl m-nitrobenzoate from the reactants methyl benzoate and nitronium ion.
Figure 3: Figure 3 shows the mechanism for the formation of methyl o-nitrobenzoate from the reactants methyl benzoate and nitronium ion.
Figure 4: Figure 4 shows the mechanism for the formation of methyl p-nitrobenzoate from the reactants methyl benzoate and nitronium ion.
Table 1: Table of Reagents Compound
Molecular Weight (g/mol)
Boiling Point (°C)
Melting Point (°C)
Density (g/ cm3)
32.04
64.7
-97.8
0.79
methyl benzoate
136.15
199.0
-12.4
1.09
methyl m-nitrobenzoate
181.15
—
78.0-80.0
—
methyl o-nitrobenzoate
181.15
—
-13.0
—
methyl p-nitrobenzoate
181.15
—
94.0-96.0
—
nitric acid
63.01
83.0
-41.6
1.51
nitronium ion
46.01
—
-9.3
—
sulfuric acid
98.07
337.0
10.3
1.83
water
18.02
100.0
0.0
1.00
methanol
Equation 1: Equation 1 shows the two calculations to determine the limiting reagent. Methyl benzoate is the limiting reagent, and 2.20x10-3 mol is the theoretical yield.
Equation 2: Equation 2 shows the calculation for the actual yield, which is 4.03x10-4.
Equation 3: Equation 3 shows the formula and calculation for percent yield which is 18.32%.
Table 3: Proton NMR Analysis for m-Nitrobenzoate Label
Multiplicity
Integration
Shift (ppm)
A
1
3
3.9
B
3
1
7.7
C
2
2
8.5
D
1
1
8.9...
Similar Free PDFs

Nitration of Methyl Benzoate
- 4 Pages

Nitration of Methyl Benzoate
- 2 Pages

Nitration of Methyl Benzoate
- 9 Pages

preparation of benzyl benzoate
- 6 Pages
Popular Institutions
- Tinajero National High School - Annex
- Politeknik Caltex Riau
- Yokohama City University
- SGT University
- University of Al-Qadisiyah
- Divine Word College of Vigan
- Techniek College Rotterdam
- Universidade de Santiago
- Universiti Teknologi MARA Cawangan Johor Kampus Pasir Gudang
- Poltekkes Kemenkes Yogyakarta
- Baguio City National High School
- Colegio san marcos
- preparatoria uno
- Centro de Bachillerato Tecnológico Industrial y de Servicios No. 107
- Dalian Maritime University
- Quang Trung Secondary School
- Colegio Tecnológico en Informática
- Corporación Regional de Educación Superior
- Grupo CEDVA
- Dar Al Uloom University
- Centro de Estudios Preuniversitarios de la Universidad Nacional de Ingeniería
- 上智大学
- Aakash International School, Nuna Majara
- San Felipe Neri Catholic School
- Kang Chiao International School - New Taipei City
- Misamis Occidental National High School
- Institución Educativa Escuela Normal Juan Ladrilleros
- Kolehiyo ng Pantukan
- Batanes State College
- Instituto Continental
- Sekolah Menengah Kejuruan Kesehatan Kaltara (Tarakan)
- Colegio de La Inmaculada Concepcion - Cebu