Orgo 2 Exp 6 - Nitration of Methyl Benzoate PDF

| Title | Orgo 2 Exp 6 - Nitration of Methyl Benzoate |
|---|---|
| Author | Maya Givens |
| Course | Organic Chemistry Laboratory II |
| Institution | University of South Florida |
| Pages | 12 |
| File Size | 534.6 KB |
| File Type | |
| Total Downloads | 82 |
| Total Views | 126 |
Summary
USF Lab Report, I earned an A for this report...
Description
University of South Florida
Experiment 6 Nitration of Methyl Benzoate
Maya Givens Jason Cuce || CHM 2211L 06 October 2020
1
Table of Contents
Table of Contents
1
Introduction
2
Chemicals
4
Experiment
5
Results
6
Discussion
8
Conclusion
9
References
11
2
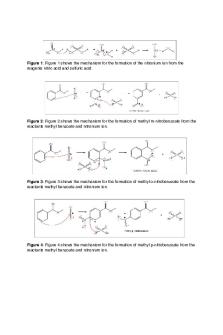
Introduction An aromatic compound is identified by planar rings and covalent bonds that provide unique standard of stability known as, aromaticity2 . This particular compound arrangement is characterized by cyclic geometry, planar symmetry, sp2 hybridized atoms that can form a delocalized system of 𝛑 molecular orbitals, and Hückel's rule where the number of electrons in the aforementioned system must equal [4n + 2]3 . Most aromatic compounds contain a benzene ring or a similar structure because benzene follows the rules from the previous list but not all cyclic compounds do. Even though there is a presence of alkenes in aromatic compounds they undergo a different typ of reaction. The delocalized system of 𝛑 molecular orbitals and high stability encourage aromatic compounds to undergo electrophilic aromatic substitution instead of an addition reaction seen in alkenes2. The reagents in this reaction are electrophilic, a 𝛑 bond within the system attacks the electrophile and then a bond will form between the aromatic and electrophile. This then determines the rate, removes aromaticity (temporarily) and an intermediate is formed known as the arenium intermediate. This intermediate will be deprotonated, the compound rearomatized, and the substituted product created. For this experiment, methyl benzoate contains 𝛑 bonds, nitric and sulfuric acid will form the electrophile; nitronium ion. One of the 𝛑 bonds will attack the nitronium ion to produce the arenium intermediate2 . The intermediate is deprotonated by water, and the product is 3-nitromethylbenzoate. Substituents on the aromatic compound are known as directors. For aromatic compounds to favor ortho (1, 2) or para (1, 4) substitution they need activating substituents4 . Activating substituents
3 such as the alkyls: NH2, NR2, OH, OCH3, OR, SR, donate electrons and make the aromatic compound more reactive3 . They are more stable in nature because of resonance. On the contrary deactivating substituents such as atoms with carbonyls, high electronegativities, or inductive effects, will favor meta substitution (1, 3). In this experiment methyl benzoate contains a meta director, COOCH3, this carbonyl group is electron withdrawing2 . Because of this the substitution cannot be ortho or para, the withdrawing group would be adjacent to the intermediate which is unfavorable. For a meta substitution like this one the major product is favored. Below is the mechanism for the desired reaction:
Figure 1: Mechanism Nitration of Methyl Benzoate
4 It is possible though unlikely for a side reaction to occur in the presence of excess NO2. Below is the proposed mechanism:
Figure 2: Possible Side Reaction Nitration of Methyl Benzoate
Chemicals Always wear PPE Chemical
Sulfuric Acid
Nitric Acid
Methyl Benzoate
Molar Mass
98.079
63.01
136.15
Boiling Point
337
83
199
Melting Point
10
-42
-15
Hazards Toxicity
Do not inhale or ingest. Corrosive to metals May cause irritation
Do not inhale or ingest. Corrosive to metals May cause irritation Extreme eye damage
May cause skin and eye irritation, may cause digestive issues, redness and pain
5
Image
Chemical
Methanol
Hazards Toxicity
Do not, inhale, ingest, or put in contact with skin or eyes. Flammable
Image
Experiment
6
Results Product appeared to be very fine white solid crystals Mass of the final desired product: .256g Melting point: 78-80°C 1
H NMR spectrum of the final desired product
CNMR Spectrum of Final Product
7 The theoretical 1H NMR spectrum of 3-nitromethylbenzoate
The theoretical CNMR spectrum of 3-nitromethylbenzoate
Calculations Limiting reagent is methyl benzoate
8
Theoretical yield: .36g Percent Yield: .181/.16 = .8839*100 = 7 1%
Discussion After performing the substitution reaction the final mass of the product was .256g and melting point ranged from 78-80°C. According to literature the melting point of
3-nitromethylbenzoate is 78°C so the observed melting point was consistent with the final product. As for the mass obtained, our reaction yielded .256g, the theoretical yield was .36g 3-nitromethylbenzoate. This gave a percentage yield of 71%, which is not necessarily high but I will discuss reasons for a lower yield in my error portion. 71% is still high enough to conclude a success upon analysis of the other data. The HNMR spectra presented a sharp peak at around 3.7ppm and another two peaks presenting to the left at around 7.7ppm and 8ppm. The theoretical HNMR presented nearly identical with a sharp peak singlet at around 3.7ppm and two other peaks also at 7.7ppm a doublet and 8ppm a triplet. The first sharp peak is indicative of the benzene sextet present in the aromatic compound. The two peaks presenting more to the left indicate the presence of
9
an aldehyde and aromatic ring with a proton (hydrogen) present. The HNMR spectra obtained is conclusive that 3-nitromethylbenzoate was our product.
Error As I did not perform the experiment I can’t pinpoint the sources of error. The largest one of note is the percentage yield, it came in low at 71%. I can attribute this to human error in transferring of the product. The reaction required the compound to be vacuum filtered twice wherein which product may have been lost. Also in transferring the product to drying paper. This is what I account for in the possible error and lower percent yield.
Conclusion The data obtained from this experiment allows me to conclude that a successful aromatic substitution reaction was performed. The HNMR spectra was practically identical to the proposed one from literature. The melting point also leads me to conclude a successful experiment as it presented at 78° C equivalent to that found in literature. The percent yield was slightly low as stated above in the discussion but was still high enough and when paired with the obtained HNMR spectra and melting point to determine a successful reaction. Based on this data I will conclude a successful nitration of methyl benzoate to 3-nitromethylbenzoate. Nitration reactions are often found in the synthesis of pharmaceuticals. Many life saving medications require the synthesis of safe intermediates. C-nitration of aromatic compounds is one of the most common found in pharmaceutical production1 . Nitroglycerin (nitro) is a vasodilator commonly used to counteract the effects of a myocardial infarction or heart attack. Nitro is a foundational medicine taught to all levels of healthcare including basic life support
10 because it’s use is so wildly effective in life saving measures. Below are a few common modifications of nitrogen containing aromatic compounds in pharmaceuticals:
Figure 3: Synthetic modifications of P-NBA
Aromatic substitution has been found to be one of the safer methods for pharmaceutical plants to synthesize these intermediates on a large scale. Nitroglycerin was one of the first to be successfully produced and it has been about 100 years of life saving affects since its artificial synthesis1. Knowing of its success has allowed such syntheses to expand, the success can be accredited to the stability of aromatic compounds and their ability to yield large amounts of the major intended product. We learn more about aromatic substitution and its application to healthcare because of such discoveries in organic chemistry.
11
References (1) Firth, D. Nitration Reactions in the Manufacture of Pharmaceutical Intermediates. http://www.iptonline.com/articles/public/IPTSEVEN132NP.pdf (accessed 2020). (2) Libretexts. 22.4: Electrophilic Aromatic Substitution. https://chem.libretexts.org/Bookshelves/Organic_Chemistry/Book:_Basic_Principles_of_ Organic_Chemistry_(Roberts_and_Caserio)/22:_Arenes_Electrophilic_Aromatic_Substit ution/22.04:_Electrophilic_Aromatic_Substitution (accessed Oct 5, 2020). (3) Ouellette, R.; Rawn, J.; Mizuhata, Y.; Tokitoh, N. Aromatic Compounds. https://www.sciencedirect.com/topics/chemistry/aromatic-compound (accessed Oct 5, 2020). (4) Weldegirma, S. Experimental Organic Chemistry , 9th ed.; University of South Florida: Department of Chemistry: Tampa, Fl, 2020....
Similar Free PDFs

Nitration of Methyl Benzoate
- 4 Pages

Nitration of Methyl Benzoate
- 2 Pages

Nitration of Methyl Benzoate
- 9 Pages

EXP 2 - Sodium Benzoate
- 4 Pages

Exp 7 orgo 2
- 10 Pages
Popular Institutions
- Tinajero National High School - Annex
- Politeknik Caltex Riau
- Yokohama City University
- SGT University
- University of Al-Qadisiyah
- Divine Word College of Vigan
- Techniek College Rotterdam
- Universidade de Santiago
- Universiti Teknologi MARA Cawangan Johor Kampus Pasir Gudang
- Poltekkes Kemenkes Yogyakarta
- Baguio City National High School
- Colegio san marcos
- preparatoria uno
- Centro de Bachillerato Tecnológico Industrial y de Servicios No. 107
- Dalian Maritime University
- Quang Trung Secondary School
- Colegio Tecnológico en Informática
- Corporación Regional de Educación Superior
- Grupo CEDVA
- Dar Al Uloom University
- Centro de Estudios Preuniversitarios de la Universidad Nacional de Ingeniería
- 上智大学
- Aakash International School, Nuna Majara
- San Felipe Neri Catholic School
- Kang Chiao International School - New Taipei City
- Misamis Occidental National High School
- Institución Educativa Escuela Normal Juan Ladrilleros
- Kolehiyo ng Pantukan
- Batanes State College
- Instituto Continental
- Sekolah Menengah Kejuruan Kesehatan Kaltara (Tarakan)
- Colegio de La Inmaculada Concepcion - Cebu