Nitration of Methyl Benzoate Lab Report PDF

| Title | Nitration of Methyl Benzoate Lab Report |
|---|---|
| Author | Ansley Morgan |
| Course | Organic Chemistry Ii |
| Institution | Middle Tennessee State University |
| Pages | 2 |
| File Size | 155.3 KB |
| File Type | |
| Total Downloads | 70 |
| Total Views | 163 |
Summary
Given by Norma Dunlap...
Description
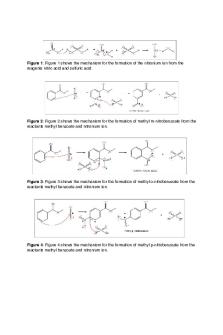
Ansley Morgan Chem 3021-001 March 13, 2019 Nitration of Methyl Benzoate Introduction Benzene does not react with most reagents like it is expected to, but in the case of the nitration reaction it reacts with electrophiles in electrophilic aromatic substitution. As stated by Ilaria Di Somma in Kinetic and Safety Characterization of the Nitration Process of Methyl Benzoate in Mixed Acid, the mixed acids using in the nitration of methyl benzoate create a very important resonance stabilized intermediate. Although the creation of this intermediate is very fascinating it ultimately has to be deprotonated in order to regenerate an aromatic ring. The goal of this experiment was to create methyl 3-nitrobenzoate from nitrating methyl benzoate. To check if we were successful a 1H NMR was analyzed. Abstract Nitration is a commonly used reaction that involves an addition reaction that results in a resonance stabilized intermediate that is later deprotonated to regenerate an aromatic ring. This reaction turned methyl benzoate into methyl 3-nitrobenzoate. Because of methyl benzoate’s substituent the nitro group is added in the meta position. The procedure included combining sulfuric acid, methyl benzoate, and nitric acid, using suction filtration, purification through recrystallization, and 1 H-NMR analysis. Discussion To start this experiment a mixture of sulfuric acid and methyl benzoate was combined with a mixture of sulfuric and nitric acids. The mixture was then warmed to room temperature and pipetted onto ice. The product was the filtered using suction and washed several times with water and ice-cold methanol. After washing, the product was crystallized from an equal weight of methanol. The resulting crystal weighed 0.084 g. A percent yield of 54% was then calculated by dividing the resulting mass of the product by the mass of the starting material. A melting point was then taken and determined to be 58 degrees Celsius. Lastly a 1H NMR was performed and gave the expected results. The mechanism of this reaction is shown below.
Experimental In this experiment methyl benzoate was nitrated to create methyl 3-nitrobenzoate. We started with combining sulfuric acid, methyl benzoate, and nitric acid. That mixture was then heated, pipetted onto ice, and filtered through a Hirsch funnel. The remaining product was then washed with water and ice-cold methanol. That product was the crystallized from an equal amount of methanol. The resulting product was weighed and a melting point was taken. A percent yield of 54% was calculated by dividing the final mass of 0.084 g by the starting mass of 0.157 g. The resulting 1H NMR is shown below. Conclusions In conclusion, this experiment involved nitrating methyl benzoate in order to make methyl 3nitrobenzoate. The combination of the three starting materials resulted in a white crystalline structure after the crystallization process. The structure was determined to be methyl 3nitrobenzoate after analyzing the NMR. This process is similar to the ones researchers are using to create explosives, solvents, and pharmaceuticals.
References Di Somma, I.; Marotta, R.; Andreozzi, R.; Caprio, V. Kinetic and Safety Characterization of the Nitration Process of Methyl Benzoate in Mixed Acid. Organic Process and Development online. 2012....
Similar Free PDFs

Nitration of Methyl Benzoate
- 4 Pages

Nitration of Methyl Benzoate
- 2 Pages

Nitration of Methyl Benzoate
- 9 Pages
Popular Institutions
- Tinajero National High School - Annex
- Politeknik Caltex Riau
- Yokohama City University
- SGT University
- University of Al-Qadisiyah
- Divine Word College of Vigan
- Techniek College Rotterdam
- Universidade de Santiago
- Universiti Teknologi MARA Cawangan Johor Kampus Pasir Gudang
- Poltekkes Kemenkes Yogyakarta
- Baguio City National High School
- Colegio san marcos
- preparatoria uno
- Centro de Bachillerato Tecnológico Industrial y de Servicios No. 107
- Dalian Maritime University
- Quang Trung Secondary School
- Colegio Tecnológico en Informática
- Corporación Regional de Educación Superior
- Grupo CEDVA
- Dar Al Uloom University
- Centro de Estudios Preuniversitarios de la Universidad Nacional de Ingeniería
- 上智大学
- Aakash International School, Nuna Majara
- San Felipe Neri Catholic School
- Kang Chiao International School - New Taipei City
- Misamis Occidental National High School
- Institución Educativa Escuela Normal Juan Ladrilleros
- Kolehiyo ng Pantukan
- Batanes State College
- Instituto Continental
- Sekolah Menengah Kejuruan Kesehatan Kaltara (Tarakan)
- Colegio de La Inmaculada Concepcion - Cebu