Organic Chem Lab 6: Nitration of Methyl Benzoate PDF

| Title | Organic Chem Lab 6: Nitration of Methyl Benzoate |
|---|---|
| Course | Organic Chem Lab |
| Institution | Howard University |
| Pages | 7 |
| File Size | 184.7 KB |
| File Type | |
| Total Downloads | 220 |
| Total Views | 572 |
Summary
Nitration of Methyl BenzoateAbstractThe objective of this experiment is to prepare methyl 3-nitrobenzoate through electrophilic aromatic substitution by nitrating methyl benzoate and analyze the products obtained using the melting point and Thin Layer Chromatography (TLC).Introduction This nitration...
Description
Nitration of Methyl Benzoate
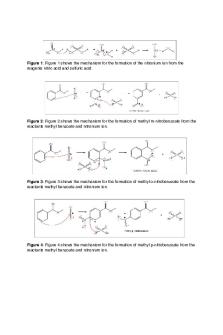
Abstract The objective of this experiment is to prepare methyl 3-nitrobenzoate through electrophilic aromatic substitution by nitrating methyl benzoate and analyze the products obtained using the melting point and Thin Layer Chromatography (TLC). Introduction This nitration of methyl benzoate is an electrophilic aromatic substitution or EAS reaction. An
electrophilic aromatic substitution is a reaction that takes place when a hydrogen atom on an aromatic ring is replaced by a positively charged ion as a result of an electrophilic attack on the ring. There are many factors that determine where the electrophile attacks and they include resonance and steric hindrance. Electrophiles can either attack at the meta, ortho, or para position depending on which position provides the least steric hindrance and encourages stability. In the nitration of methyl benzoate, the electrophile that attacks the aromatic ring is formed from the mixture of sulfuric and nitric acid. This mixture produces the nitronium ion (NO ), which is the active species that attacks the aromatic ring. In this reaction nitration at the + 2
meta position is favored because the partial positive charges that reside at the ortho and para positions repel the positively charged nitronium ion. Table1. Physical and Chemical Properties of Chemicals Used Throughout the Experiment Compound
Molecular Weight (g/mol)
Density (g/mL)
Melting Point (°C)
Boiling Point (°C)
Methyl Benzoate
136.15
1.087
-13.0
198-199
Methyl 3-nitrobenzoate
181.15
1.301
78
279
Experimental Procedure: Six milliliters of concentrated sulfuric acid was transferred into a 125-mL Erlenmeyer flask and cooled to 0°C in an ice bath. Three grams of methyl benzoate was then added to the cold sulfuric acid. The combined mixture was cooled to about 0-10°C. In an Erlenmeyer flask, a mixture containing 2 mL of concentrated sulfuric acid and 2 ml of concentrated nitric acid was cooled in an ice bath. The cooled nitric and sulfuric acid was then added dropwise to the solution of
sulfuric acid and methyl benzoate. During this process, the temperature was monitored and kept below 15°C. After the nitric and sulfuric acid was added, the reaction mixture was allowed to warm up to room temperature for 15 minutes after being removed from the ice. After the reaction mixture was warmed to room temperature, it was poured over 25 grams of cracked ice in a 100 mL beaker. The solid product was isolated using vacuum filtration. The solid was washed with ice-cold water and two parts of 7ml of ice-cold methanol. The crude product was allowed to air dry. Once dry, the remaining product was weighed and a small sample was saved to conduct the melting point and TLC. The amount of crude product left was then add an equal amount of methanol in a small test tube. Using a hot water bath with a temperature of 70°C the remaining product was heated until completely dissolved. Using vacuum filtration, the recrystallized product was isolated. The crystals were washed with ice-cold methanol and allowed to air dry. The melting points of the crude and recrystallized product were determined. A TLC was performed on the crude and recrystallized product as well.
Chemical reaction:
Formation of nitrating reagent + HNO3 + 2H2SO4 ↔ NO2+ + 2 H2SO 4-+H 3O Formation of methyl 3-nitrobenzoate C6H5CO2CH3 (HNO3→ H2SO4) → C8H7NO4
Results: Yield Report Weight of methyl benzoate
3g
Moles of methyl benzoate
0.02 mol
Volume of nitric acid Moles of nitric acid Volume of sulfuric acid
2 mL 0.002 mol 2 mL
Moles of sulfuric acid
0.002 mol
Theoretical yield of methyl 3-nitrobenzoate
0.02 mol
Theoretical yield of methyl 3-nitrobenzoate
3.99 g
Actual yield of recrystallized methyl 3-nitrobenzoate
1.76g
Percent yield of methyl 3 nitrobenzoate
44.1%
Melting point(range) of crude product
69.4 -78.1°C
Melting point(range) of recrystallized product
77.4-81.2°C
Calculation of Theoretical yield 3 g methyl benzoate x
1 mol methyl benzoate 136.15 g methyl benzoate
= 0.02 mol methyl benzoate (limiting reagent)
1:1 ratio of reactants to products, therefore, 0.02 moles of methyl 3-nitrobenzoate will be produced. Theoretical yield of methyl 3-nitrobenzoate = 0.02moles x 181.5g/mol = 3.99g % Yield of methyl 3-nitrobenzoate = 1.76g/3.99 g X 100 = 44.1% Thin Layer Chromatography Results: Product
Rf
Crude methyl 3-nitrobenzoate
0.84
Recrystallized methyl 3-nitrobenzoate
0.84
Original methyl benzoate
0.90
Discussion:
The objective of this experiment was to prepare methyl 3-nitrobenzoate through electrophilic aromatic substitution by nitrating methyl benzoate. By analyzing the melting point of the obtained products and using thin layer chromatography we were able to confirm the successful formation of methyl 3-nitrobenzoate.
The melting point obtained for both the crude and the recrystallized product fell within the range of the expected melting point. The expected melting point for methyl 3-nitrobenzoate was 78 °C. The melting point of the crudemethyl 3-nitrobenzoate was in the range of 69.4 -78.1°C and the recrystallized methyl 3-nitrobenzoate was in the range 77.4-81.2°C. Although there was a slight difference, both the crude and the recrystallized prodcts’ melting point was close to the expected 78 °C. From these observed melting points it can be assumed that the correct product was formed during this experiment.
Thin Layer Chromatography was used to assess the purity of a compound. Three spots
were placed onto the plate, methyl benzoate, the crude product, and the recrystallized product. There were three spots that were visualized on the plate. The R values were calculated and f
determined that the substance was pure because the crude and the recrystallized R values were f
very close to each other suggesting purity. The R value for the products obtained were also f
different from the starting product of pure methyl benzoate indicating that methyl 3-nitrobenzoate was successfully formed in the reaction.
Although methyl 3-nitrobenzoate was successfully obtained, the percent yield of the recrystallized methyl 3-nitrobenzoate was 44%, this low yield can be due to incomplete isolation
as some of the product could have remained in the reaction with the methanol while it was being dissolved.
Conclusion:
The results obtained support that the product formed, methyl 3-nitrobenzoate, was obtained from nitrating methyl benzoate. This nitration took place via an electrophilic aromatic substitution and the electrophile was formed from mixing sulfuric and nitric acid. Although the yield of the product was lower than expected, TLC and melting point analysis was able to confirm the successful formation of methyl 3-nitrobenzoate....
Similar Free PDFs

Nitration of Methyl Benzoate
- 4 Pages

Nitration of Methyl Benzoate
- 2 Pages

Nitration of Methyl Benzoate
- 9 Pages

Lab 6 Nitration LAB
- 5 Pages
Popular Institutions
- Tinajero National High School - Annex
- Politeknik Caltex Riau
- Yokohama City University
- SGT University
- University of Al-Qadisiyah
- Divine Word College of Vigan
- Techniek College Rotterdam
- Universidade de Santiago
- Universiti Teknologi MARA Cawangan Johor Kampus Pasir Gudang
- Poltekkes Kemenkes Yogyakarta
- Baguio City National High School
- Colegio san marcos
- preparatoria uno
- Centro de Bachillerato Tecnológico Industrial y de Servicios No. 107
- Dalian Maritime University
- Quang Trung Secondary School
- Colegio Tecnológico en Informática
- Corporación Regional de Educación Superior
- Grupo CEDVA
- Dar Al Uloom University
- Centro de Estudios Preuniversitarios de la Universidad Nacional de Ingeniería
- 上智大学
- Aakash International School, Nuna Majara
- San Felipe Neri Catholic School
- Kang Chiao International School - New Taipei City
- Misamis Occidental National High School
- Institución Educativa Escuela Normal Juan Ladrilleros
- Kolehiyo ng Pantukan
- Batanes State College
- Instituto Continental
- Sekolah Menengah Kejuruan Kesehatan Kaltara (Tarakan)
- Colegio de La Inmaculada Concepcion - Cebu