Experiment 6: Nitration of Methyl Benzoate PDF

| Title | Experiment 6: Nitration of Methyl Benzoate |
|---|---|
| Author | Brenda Hernandez |
| Course | Organic Chemistry Laboratory II |
| Institution | University of South Florida |
| Pages | 13 |
| File Size | 410.1 KB |
| File Type | |
| Total Downloads | 43 |
| Total Views | 145 |
Summary
I received a 40/40 for this report for some reason....
Description
Experiment Six: Nitration of Methyl Benzoate TA: Ning Shen February 24, 2021
Introduction: Background: In experiment six: Nitration of Methyl Benzoate, students were to produce methyl 3-nitro benzoate through the nitration of methyl benzoate. 1 This nitration reaction is an example of an electrophilic substitution reaction of an aromatic compound, methyl benzoate. 1 To understand this reaction, one must understand what aromatic compounds are. For a compound to be considered an aromatic, they must fulfill two general requirements. 2 One, the compound must be cyclic. Two, the number of pi electrons must follow Huckel’s rule, 4n+2 (where n = an integer).2 Other important things to keep in mind are that aromatic compounds have no sp3 hybridization and must be planar.2 Based on what substituent is connected to the ring, the aromatic is categorized as either an activating ring (where the substituent donates electron) or a deactivating ring (where the substituent withdraws electrons).3 Common activating groups include: NH 2, NR2, OH, OR, Methyl, and alkyl groups.3 Common deactivating groups include: NO 2, CF3, COR, CO2R, CN, halogens, and SO3H.3 Depending on what type of substituent group is attached to the aromatic ring, it will cause the reaction to go in a specific direction.3 An activating group will act as an ortho or para director.3 For ortho-directors, substitution will occur at carbons 2 and/or 6. 3 For para-directors, substitution will occur at carbon 4. 3 Deactivating groups will act as meta directors, where substitution will occur at carbons 3 and/or 5.3 Electrophilic aromatic substitution reactions are usually used to produce another aromatic ring.4 There are five different electrophilic aromatic substitution reactions: halogenation,
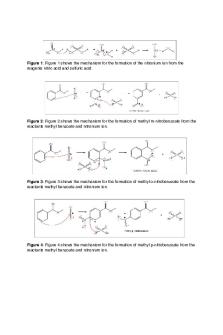
nitration, sulfonation, alkylation, and acylation Friedel-Crafts. 4 Both alkylation and acylation happen through a Friedel-Crafts reaction.4 During nitration, an aromatic compound, such as methyl benzoate, is reacted with nitric acid, heat, and a catalyst.4 Nitration specifically asks that the catalyst be sulfuric acid so that when combined with nitric acid, it will produce an electrophilic nitronium ion (as seen in figure 1).1 As seen in figure 2, this nitronium ion reacts with methyl benzoate to produce a sigma complex. This intermediate then reacts with hydrogen sulfate to finally produce Methyl 3Nitrobenzoate and sulfuric acid. The ester found in methyl benzoate is a deactivating group which causes acts as a meta-director; hence our reaction produced Methyl 3-Nitrobenzoate, a meta-product.3
Mechanism:
Figure 1: The Creation of the Nitronium Ion generated by Nitric Acid and Sulfuric Acid.
O
Figure 2: The Mechanism of the Nitration of Methyl Benzoate.
Side Reactions:
Figure 3: Side Reaction when Excess Acid and Water Interacts Methyl 3-Nitrobenzene. To prevent this from happening, avoid excess water.
Figure 4: Unlikely Side Reaction when Methyl 3-Nitrobenzoate interacts with Nitric Acid and Sulfuric Acid.
Experimental:
MEDIUM TEST TUBE
Weigh the product, transfer to clean test tube, recrystallize from equal weight of MeOH, heat mix to dissolve minimum amount of solvent, remove sample from heat, cool to room temperature & then 0 ℃ to form crystals, and filter the product.
3-NITROMETHYL BENZOATE
Add 0.3mL concentrated H2 SO 4, 0.3g of methyl benzoate, swirl mixture, cool to 0 ℃, prepare 0.2mL H2 SO4 & 0.2 mL HNO 2 mixture, add mixture to test tube dropwise while stirring 2-5 min. while Keeping Rxn at 0℃
REACTION MIXTURE AT ROOM TEMPERATURE
CRUDE PRODUCT CRYSTALLIZATION
Remove the mixture from Ice & earm to RT for 15 minutes, 3g racked ice to a 50-mL beaker, pour mix into cracked ice, product should solidify, vacuum filtration, wash with cold H2O and cold CH 4O.
ANALYSIS
Obtain mass, calculate % yield, determine melting point, obtain 1H NMR & 13C NMR, and complete questions within manual.
END
Chemicals Used: Chemicals: Sulfuric Acid (H2SO4)
Physical Properties: Molar Mass: 98.079g/mol Melting Point: 10℃ Boiling Point: 337℃
Chemical Properties: Extremely corrosive May cause severe burns May cause eye/skin irritation
Oxidane (H2O)
Molar Mass: 18.015g/mol Melting Point: 0℃ Boiling Point: 100℃
May cause severe burns
Methanol (CH4O)
Molar Mass: 32.04g/mol Melting Point: 97.6℃ Boiling Point: 64.7℃
Toxic if inhaled/ingested May cause damage to organs Extremely flammable May cause eye/skin irritation Extremely corrosive
Nitric Acid (HNO3)
Methyl 3-Nitrobenzoate (C8H7NO4)
Methyl Benzoate (C8H8O2)
Molar Mass:63.01g/mol Melting Point: -42℃ Boiling Point: 83℃
Molar Mass: 181.15g/mol Melting Point: 78℃ Boiling Point: 279℃
Molar Mass: 136.15g/mol Melting Point: -15℃ Boiling Point: 199℃
Avoid inhaled/ingested May cause damage to organs
Avoid ingested May cause damage to organs
Results: Appearance of the Final Product: Mass of the Starting Product (Methyl Benzoate): Mass of the Final Product (Methyl 3Nitrobenzoate): Melting Point of the Final Product: Percent Yield of the Final Product: Rate of the Reaction:
White crystals 0.3g 0.256g 78-80℃ 63.64% Relatively fast
Figure 4: Theoretical 1H NMR Spectrum for Methyl 3-Nitrobenzoate
Figure 5: The 1H NMR Spectrum Produced by the Final Product (Methyl 3-Nitrobenzoate). Calculations: Calculating the Theoretical Yield:
0.3g of C8H8O2 ×
1mol C 8 H 7 NO 4 1 mol C 8 H 8 O2 × 136.15 g C 8 H 8 O 2 1 mol C 8 H 8 O 2
= 0.0022 moles of C8H7NO4
Calculating the Actual Yield:
0.256g of C8H7NO4 ×
1 mol C 8 H 7 NO 4 = 0.0014 moles of C8H7NO4 181.15 g C 8 H 7 NO 4
Calculating the Percent Yield:
% Yield =
Actual Yield ×100 % Theoretical Yield
% Yield =
0.0014 moles C 8 H 7 NO 4 ×100 % 0.0022 moles C 8 H 7 NO 4
% Yield = 63.64%
Discussion: While conducting the experiment, students determined that the melting point of the Methyl 3-Nitrobenzoate product was between 78℃ and 80℃ which is also the theoretical melting point for said compound. Although students did not obtain the ideal product yield of 100%, they were able to produce 63.64% yield. Ideally, when one is using 1H NMR, one would be able to determine whether the expected product was obtained by comparing the proton signals of the experimental to the theoretical 1H NMR spectrum. For example, if a compound with only one proton signal is expected have a peak at 7.5ppm and the experimental 1H NMR produces a signal peak at 7.5ppm, one could conclude that the compound produced is the expected compound. In this lab, to easily distinguish whether one is observing the 1H NMR of Methyl Benzoate or Methyl 3Nitrobenzoate, one would have to check the number of peaks there are in the product’s 1H NMR. If there are four peaks, you would have produced Methyl 3-Nitrobenzoate since it has four different hydrogens which would produce a peak each (for a total of four peaks). If there are three peaks, you would have remained with the starting material, Methyl Benzoate, which only has three different types of hydrogens that would produce a peak each. Comparing both Methyl 3-Nitrobenzoate’s theoretical and experimental 1H NMR spectra, students were able to note a similar tall peak at 3.9ppm. The biggest difference is that the two peak clusters expected for said compound were found further downfield in the theoretical 1H NMR spectrum than the experimental. Specifically, the theoretical spectrum showed one cluster between 7.5ppm and 7.7ppm. The other cluster was found between 8.0ppm and 8.4ppm. In the experimental spectrum, one cluster peak was found between 7.1ppm and 7.5ppm and the other is found between 7.9ppm and 8.1ppm.
Conclusion: After comparing both the experimental and theoretical 1H NMR spectra of 3-Nitromethyl Benzoate, students observed there were only three peaks found in the product’s 1H NMR spectrum. Since students expected four signals, the spectrum did not help to clearly identify the reaction’s product. Other data obtained, such as the melting point of the product (78 ℃ -80 ℃), aided students in identifying the final product as Methyl 3-Nitrobenzoate. By reproducing the nitration of methyl benzoate and obtaining the expected product, students were able to deem this experiment successful. This experiment allowed students to further understand how 1H NMR will help identify compounds by the signals their protons give. Students were also able to research how this process can apply to other areas. For example, the nitration of ammonium is used to produced ammonium nitrate which can be used as a fertilizer. 5 Once again, organic chemistry can be applied in many areas, so it is important one understands it to put it into practice.
References: [1] Wildegirma, S. Experimental Organic Chemistry Lab Manual; University of South Florida: Tampa, FL, 2020; P. 100-101. [2] Klein, D. Organic Chemistry; Wiley & Sons: New York, 2016; p 764. [3]
LibreTexts.
Substitution
Reactions
of
Benzene
Derivatives.
https://chem.libretexts.org/Bookshelves/Organic_Chemistry/Supplemental_Modules_ %20%20%20(Organic_Chemistry)/Arenes/Reactivity_of_Arenes/Substitution_Reactions _of_Benzene_Derivatives (accessed Feb 23, 2021). [4]
Reusch,
W.
Electrophilic
Aromatic
Substitutions.
https://chem.libretexts.org/Bookshelves/Organic_Chemistry/Supplemental_Modules_(Or ganic_Chemistry)/Arenes/Reactivity_of_Arenes/Benzene/Electrophilic_Aromatic_Substi tution (accessed Feb 23, 2021). [5] Kulkarni, A. A. Continuous flow nitration in miniaturized devices. Beilstein J. Org. Chem. [online] 2014. 10, 405-424. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3943559/ (accessed Feb 23, 2021)....
Similar Free PDFs

Nitration of Methyl Benzoate
- 4 Pages

Nitration of Methyl Benzoate
- 2 Pages

Nitration of Methyl Benzoate
- 9 Pages

Lab 6 Nitration LAB
- 5 Pages
Popular Institutions
- Tinajero National High School - Annex
- Politeknik Caltex Riau
- Yokohama City University
- SGT University
- University of Al-Qadisiyah
- Divine Word College of Vigan
- Techniek College Rotterdam
- Universidade de Santiago
- Universiti Teknologi MARA Cawangan Johor Kampus Pasir Gudang
- Poltekkes Kemenkes Yogyakarta
- Baguio City National High School
- Colegio san marcos
- preparatoria uno
- Centro de Bachillerato Tecnológico Industrial y de Servicios No. 107
- Dalian Maritime University
- Quang Trung Secondary School
- Colegio Tecnológico en Informática
- Corporación Regional de Educación Superior
- Grupo CEDVA
- Dar Al Uloom University
- Centro de Estudios Preuniversitarios de la Universidad Nacional de Ingeniería
- 上智大学
- Aakash International School, Nuna Majara
- San Felipe Neri Catholic School
- Kang Chiao International School - New Taipei City
- Misamis Occidental National High School
- Institución Educativa Escuela Normal Juan Ladrilleros
- Kolehiyo ng Pantukan
- Batanes State College
- Instituto Continental
- Sekolah Menengah Kejuruan Kesehatan Kaltara (Tarakan)
- Colegio de La Inmaculada Concepcion - Cebu