Nitration Methyl benzoate Pre Lab Report PDF

| Title | Nitration Methyl benzoate Pre Lab Report |
|---|---|
| Course | Organic Chemistry II |
| Institution | University of South Florida |
| Pages | 3 |
| File Size | 162.8 KB |
| File Type | |
| Total Downloads | 6 |
| Total Views | 146 |
Summary
The Nitration of Methyl benzoate Pre Laboratory Report USF CHM2211L...
Description
Nesly Garcia CHM2211L-606 September 24,2021 Nitration of Methyl Benzoate I.
Introduction:
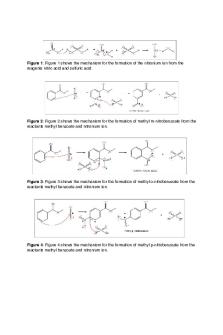
The objective of this experiment is to use methyl benzoate to produce 3-nitro methyl benzoate via a nitration reaction. Methyl benzoate nitration is considered an aromatic electrophilic substitution reaction, which is produced by nitric and sulfuric acid reacting. The substitution requires a catalyst and will substitute the electrophile for a proton on a benzene ring and produce a positively charged electrophile. In this reaction the nitronium ion is the electrophile which reacts with the intermediate that is protonated. The contact between nitronium and the intermediate will occur where the electron density is the greatest, the meta position. At this position, there is no positive resonance. Hence, the intermediate (arenium ion) forms that will have 4 resonance forms. The intermediate will pass a proton to the bisulfate ion which will yield methyl 3-nitrobenzoate. The hypothesis is that an electrophilic aromatic substitution reaction will occur by using methyl benzoate to yield 3-nitro methyl benzoate.
Figure 1. Electrophilic Aromatic Substitution Mechanism
1
II.
Materials:
1. 2 medium sized test tube 2. 0.8mL sulfuric acid(concentrated) 3. 0.3g methyl benzoate 4. 0.2mL concentrated nitric acid 5. Pasteur pipette 6. Ice 7. Stirring rod 8. 50mL beaker 9. 3g cracked ice 10. Vacuum filtration (Hirsch funnel) 11. Cold water 12. 0.5mL ice-cold methanol 13. Scale 14. Melting point apparatus III.
Procedure: 1. In a medium sized test tube, add 0.6mL of sulfuric acid and 0.3g of methyl benzoate. Mix the chemicals by swirling and allow it to reach zero degrees Celsius. Create a solution mix of 0.2mL sulfuric acid and 0.2mL nitric acid. Then, add the solution to the tube utilizing a Pasteur pipette. 2. Place the mixture in an ice bath. Then, with a stirring rod continuously mix the solution and make sure the temperature stays cooled. 3. Continue mixing while the test tube is in the ice for two minutes. Remove the test tube from the ice and allow it to return to room temperature (15 minutes). 4. Use a 50mL beaker and place the solution from the test tube in the beaker, which should have about 3 grams of cracked ice. 5. Use a Hirsch funnel for vacuum filtration for isolation of the solid product. Then, use ice cold water to wash the solid as well as 0.5mL of methanol (ice-cold). 6. Use the scale to weigh the product. Then, place it in the other test tube. 7. Crystallize the solid again from methanol which should be of equal weight. 8. Then, use filtration to find the mass of the solid and find the percent yield. 2
Pre-Lab Questions In a medium sized test tube, add 0.6mL of sulfuric acid and 0.3g of methyl benzoate. Mix the chemicals by swirling and allow it to reach zero degrees celcius.
Use a Hirsch funnel for vacuum filtration for isolation of the solid product. Then, use ice cold water to wash the solid as well as 0.5mL of methanol (ice-cold).
Use the scale to weigh the product. Then, place it in the other test tube.
Create a solution mix of 0.2mL sulfuric acid and 0.2mL nitric acid. Then, add the solution to the tube utilizing a Pasteur pipette.
Use a 50mL beaker and place the solution from the test tube in the beaker, which should have about 3 grams of cracked ice.
Crystallize the solid again from methanol which should be of equal weight.
Place the mixture in an ice bath. Then, with a stirring rod continuously mix the solution and make sure the temperature stays cooled.
Continue mixing while the test tube is in the ice for two minutes. Remove the test tube from the ice and allow it to return to room temperature (15 minutes).
Then, use filtration to find the mass of the solid and find the percent yield.
References 1. Learner, Chemistry. “Electrophilic Aromatic Substitution: Definition, Examples & Mechanism.” Chemistry Learner, 4 June 2021, www.chemistrylearner.com/electrophilicaromatic-substitution.html. 2. Reusch, William. “Aromatic Substitution Reactions.” Aromatic Reactivity, www2.chemistry.msu.edu/faculty/reusch/virttxtjml/benzrx1.htm.
3...
Similar Free PDFs

Nitration of Methyl Benzoate
- 4 Pages

Nitration of Methyl Benzoate
- 2 Pages

Nitration of Methyl Benzoate
- 9 Pages

Lab 6 Nitration LAB
- 5 Pages

Pre Lab 2 - Pre lab report 2
- 3 Pages
Popular Institutions
- Tinajero National High School - Annex
- Politeknik Caltex Riau
- Yokohama City University
- SGT University
- University of Al-Qadisiyah
- Divine Word College of Vigan
- Techniek College Rotterdam
- Universidade de Santiago
- Universiti Teknologi MARA Cawangan Johor Kampus Pasir Gudang
- Poltekkes Kemenkes Yogyakarta
- Baguio City National High School
- Colegio san marcos
- preparatoria uno
- Centro de Bachillerato Tecnológico Industrial y de Servicios No. 107
- Dalian Maritime University
- Quang Trung Secondary School
- Colegio Tecnológico en Informática
- Corporación Regional de Educación Superior
- Grupo CEDVA
- Dar Al Uloom University
- Centro de Estudios Preuniversitarios de la Universidad Nacional de Ingeniería
- 上智大学
- Aakash International School, Nuna Majara
- San Felipe Neri Catholic School
- Kang Chiao International School - New Taipei City
- Misamis Occidental National High School
- Institución Educativa Escuela Normal Juan Ladrilleros
- Kolehiyo ng Pantukan
- Batanes State College
- Instituto Continental
- Sekolah Menengah Kejuruan Kesehatan Kaltara (Tarakan)
- Colegio de La Inmaculada Concepcion - Cebu