Nitration of Methyl Benzoate Lab Report PDF

| Title | Nitration of Methyl Benzoate Lab Report |
|---|---|
| Course | Organic Chemistry II Lab |
| Institution | University of Alabama at Birmingham |
| Pages | 9 |
| File Size | 319.3 KB |
| File Type | |
| Total Downloads | 38 |
| Total Views | 153 |
Summary
Nitration of Methyl Benzoate Lab Report with a 100%...
Description
Nitration of Methyl Benzoate Writer - Matthew Estacio Reviewer - Maddie Looney Editor - Nora Cipkowski
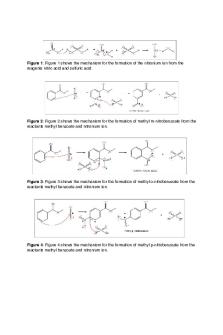
Introduction Nitration is considered to be one of the most important methods of electrophilic aromatic substitution. Products such as explosives and pharmaceutical synthetic intermediates contain aromatic nitro compounds. Nitration involves the addition of nitrogen to a benzene ring to form nitroaromatic compounds such as nitrobenzene, and this ring can act as a reagent in additional substitution reactions. Mechanism The nitronium ion NO2+ acts as the electrophile in nitration reactions. Protonation and loss of water produce the nitronium ion from concentrated nitric acid (HNO3), using concentrated sulfuric acid (H2SO4) as the dehydration agent (Figure 1).
Figure 1: Figure 1 depicts the formation of the nitronium ion. During the nitration of an unsubstituted benzene, the ring attacks the nitronium ion. This causes a transient disruption of the aromaticity of the ring, forming a cationic intermediate (Reference 1). Three resonance structures stabilize the intermediate by spreading the positive charge over the ring. Eventually, nitrobenzene is formed as the ring re-aromatizes (Figure 2).
Figure 2: Figure 2 depicts the three resonance structures of the cationic intermediate. In this experiment, a reaction occurred between methyl benzoate and the nitronium ion. Three mono-nitrated products could possibly form: ortho-nitromethyl benzoate, metanitromethyl benzoate, and para-nitromethyl benzoate (Figure 3).
Figure 3: Figure 3 depicts the three possible mono-nitrated products: ortho-nitromethyl benzoate, meta-nitromethyl benzoate (Reference 2), and para-nitromethyl benzoate (left to right). Table 1: Table of Reagents Compound
MW (g/mol)
BP (℃)
MP (℃)
Density (g/mL)
Methanol
32.04
64.7
-97.6
0.791
Methyl benzoate
136.15
199
-15
1.08
Nitric acid
63.01
83
-42
1.51
Sulfuric acid
98.079
337
10
1.83
Water
18.01528
100
0
1.00
Ortho-nitromethyl benzoate
181.15
-
-13
-
Meta-nitromethyl benzoate
181.15
-
78-80
-
Para-nitromethyl benzoate
181.15
-
94-96
-
Experimental 0.6 mL of sulfuric acid was added to an Erlenmeyer flask and placed into an ice bath. Following this, 0.3 mL of methyl benzoate was added to the flask. 0.2 mL of both sulfuric acid and nitric
acid were added to a vial and then placed in an ice bath. The acid solution was added drop by drop to the methyl benzoate solution while it was mixed and kept cold in an ice water bath. Then, ice was added to the Erlenmeyer flask. To separate the precipitate, a Hirsch funnel was used to vacuum out the excess (Reference 3). The solid was washed with 1 mL of cold water and then 1 mL of methanol two separate times to ensure as much of the product was retrieved as possible. The precipitate was vacuumed until it was dry. Finally, the mass, melting point, and NMR was taken of the final product.
Results Starting volume: 0.30 mL Final mass: 0.04 g Melting point: 76.2-78.1℃ This experiment involved the reaction between methyl benzoate and nitric acid, which formed methyl nitrobenzoate. When everything was added to the flask, the solution was translucent and a light orange color, which indicated the nitration reaction occurring. The precipitate started to form when ice was added, and the ice was allowed to melt. The solid product was an off-white color. Some of the product was left in the flask during filtration, so 1 mL of water was added to loosen it up so that it could be poured out. The percent yield of the product was calculated, and the melting point was obtained. To calculate the percent yield, the limiting reagent was determined first (Equation 1). Eq. 1
mass (g) / MW (g/mol) = mol product
Methyl benzoate: (0.3 mL)(1.08 g/mL) / (136.15 g/mol) = 0.00238 mol Nitric acid: (0.2 mL)(1.51 g/mL) / (63.01 g/mol) = 0.00479 mol
Methyl benzoate was determined to be the limiting reagent, and the theoretical yield of the product was then determined (Equation 2). Eq. 2
mol product x product MW (g/mol) = theoretical yield (g) (0.00238 mol) x (181.15 g/mol) = 0.431 g
The percent yield of the product was then calculated (Equation 3). Eq. 3
(actual yield / theoretical yield) x 100 = percent yield (0.04 g / 0.431 g) x 100 = 9.28% yield
Discussion In this experiment, methyl nitrobenzoate was produced by the reaction between methyl benzoate and nitric acid. After adding everything to the flask, the solution was translucent and light orange in color. This was indicative that the nitration reaction was occurring. When ice was added, the precipitate started to form, and the ice was then allowed to melt. The precipitate was an off-white color. 1 mL of water was added to the flask to loosen up some of the product that was stuck inside. This enabled the rest of the product to be poured out. Methyl benzoate acted as the nucleophile in this experiment. The nitronium ion acted as the electrophile in the reaction and was formed by nitric acid. Nitric acid received a proton from sulfuric acid, which caused the nitronium ion to form. To aid in product precipitation, ice was used. To purify the final solid product, methanol and water were used. Nitric acid could possibly add to one of three positions: ortho, meta, or para. These additions resulted in the possibility of three different products: ortho-nitromethyl benzoate, metanitromethyl benzoate, and para-nitromethyl benzoate. Electron-withdrawing groups have a deactivating effect on the ring. The ester group attached to the benzene ring of methyl benzoate
is an electron-withdrawing group and thus a meta-director. Attaching to the meta position provides a more stable secondary carbocation intermediate in comparison to the ortho and para positions. This implied that meta-nitromethyl benzoate would be the major product. The percent yield of the product was calculated to be 9.28%. This low percent yield could have occurred as a result of spilling the reagents during the addition of each or unreacted reagents. The melting point of the product was 76.2 - 78.1°C. This melting point value closely matches the literature melting point value of meta-nitromethyl benzoate, which is 78-80℃. The slightly lower observed melting point could have occurred as a result of unreacted reagents. All of the reagents had significantly lower melting points. Since the melting point was only slightly lower, this indicated that the product was relatively pure.
Conclusion Meta-nitrobenzoate was the major product formed in this experiment. According to the melting point that was obtained at 76.2 - 78.1 °C, the final product was meta-nitromethyl benzoate. Although the percent yield was low, according to the accurate melting point and H-NMR data, the product had a high level of purity. In order to improve the purity and yield of this product and the experiment overall, the reaction should be kept in an ice bath consistently throughout the experiment. This will prevent several different side reactions from occurring.
References
1
Anasazi Experiment Series. (n.d.). Electrophilic Aromatic Substitution: Nitration of Methyl Benzoate. Retrieved April 13, 2021, from https://www.aiinmr.com/wp-content/uploads/2020/01/Electrophilic-Aromatic-Substitutio n-Nitration-of-Methyl-Benzoate.pdf
2
National Center for Biotechnology Information (2021). PubChem Compound Summary for CID 69260, Methyl 3-nitrobenzoate. Retrieved April 13, 2021 from https://pubchem.ncbi.nlm.nih.gov/compound/Methyl-3-nitrobenzoate.
3
Vacuum filtration. (2007, August 8). Retrieved April 13, 2021, from http://www.chem.ucla.edu/~bacher/General/30BL/tips/vacuum
Complete NMR
Figure 4: Figure 4 depicts the complete H-NMR of m-nitromethyl benzoate. Table 2: H-NMR of m-Nitromethyl Benzoate Multiplicity
Integration
Shift
singlet
3.2076
4.0
triplet
1.0562
7.7
doublet
2.0397
8.4
singlet
1.000
8.9
The singlet at 4.0 ppm is the most shielded, followed by the triplet at 7.7 ppm. The doublet at 8.4 ppm represents the two neighboring hydrogens adjacent to the triplet. The singlet at 8.9 ppm is the most deshielded, indicating that it is between the nitro group and ester group. Both the triplet at 7.7 ppm and the singlet at 8.9 ppm indicate that meta-nitromethyl benzoate was the major product that formed. The impurity at 7.3 ppm indicates the presence of benzene. The impurity at 1.6 ppm indicates the presence of water. The impurity at 0 ppm indicates the presence of silicone grease, most likely as a result of using an unclean flask. NMR Zoomed in at the Aromatic Region
Figure 5: Figure 5 depicts H-NMR of m-nitromethyl benzoate zoomed in at the aromatic region. The triplet at 7.7 ppm is the most shielded of the three peaks in the aromatic region. The doublet at 8.4 ppm represents the two neighboring hydrogens on benzene that are adjacent to the triplet and is the second-most shielded of the three peaks. The singlet at 8.9 ppm is the most deshielded, and this indicates its location between the nitro group and ester group attached to benzene. The triplet at 7.7 ppm as well as the singlet at 8.9 ppm both indicate that the major product formed was meta-nitromethyl benzoate.
Figure 6: Figure 6 depicts m-nitromethyl benzoate with labeled peaks....
Similar Free PDFs

Nitration of Methyl Benzoate
- 4 Pages

Nitration of Methyl Benzoate
- 2 Pages

Nitration of Methyl Benzoate
- 9 Pages
Popular Institutions
- Tinajero National High School - Annex
- Politeknik Caltex Riau
- Yokohama City University
- SGT University
- University of Al-Qadisiyah
- Divine Word College of Vigan
- Techniek College Rotterdam
- Universidade de Santiago
- Universiti Teknologi MARA Cawangan Johor Kampus Pasir Gudang
- Poltekkes Kemenkes Yogyakarta
- Baguio City National High School
- Colegio san marcos
- preparatoria uno
- Centro de Bachillerato Tecnológico Industrial y de Servicios No. 107
- Dalian Maritime University
- Quang Trung Secondary School
- Colegio Tecnológico en Informática
- Corporación Regional de Educación Superior
- Grupo CEDVA
- Dar Al Uloom University
- Centro de Estudios Preuniversitarios de la Universidad Nacional de Ingeniería
- 上智大学
- Aakash International School, Nuna Majara
- San Felipe Neri Catholic School
- Kang Chiao International School - New Taipei City
- Misamis Occidental National High School
- Institución Educativa Escuela Normal Juan Ladrilleros
- Kolehiyo ng Pantukan
- Batanes State College
- Instituto Continental
- Sekolah Menengah Kejuruan Kesehatan Kaltara (Tarakan)
- Colegio de La Inmaculada Concepcion - Cebu